Outline
Risk Factors for Postmolar Gestational Trophoblastic Neoplasia
Prophylactic Chemotherapy After Molar Evacuation
Gestational Trophoblastic Neoplasia
“Phantom” Human Chorionic Gonadotropin
Treatment of Nonmetastatic and Low-Risk Metastatic Gestational Trophoblastic Neoplasia
High-Risk Metastatic Gestational Trophoblastic Neoplasia
Placental Site Trophoblastic Tumor
Coexistence of Normal Pregnancy and Gestational Trophoblastic Neoplasia
Transplacental Fetal Metastases
Survivorship Issues After Successful Treatment of Gestational Trophoblastic Neoplasia
Key Points
- 1.
Gestational trophoblastic disease (GTD) describes a spectrum of neoplastic conditions derived from the placenta, including hydatidiform moles, postmolar gestational trophoblastic neoplasia (GTN), gestational choriocarcinoma, placental site trophoblastic tumor (PSTT), and epithelioid trophoblastic tumor (ETT).
- 2.
GTN is the most curable gynecologic malignancy.
- 3.
Complete hydatidiform moles are completely derived from the paternal genome and most frequently have a diploid 46XX karyotype.
- 4.
Partial moles are composed of both paternal and maternal genomes, resulting in triploidy, and most frequently have the 69XXX karyotype. Partial moles have evidence of fetal development, which differentiates them from complete moles.
- 5.
Malignant GTN is more common after a complete molar pregnancy than a partial molar pregnancy.
- 6.
Human chorionic gonadotropin (hCG) values should be followed for 6 months after molar pregnancy evacuation to detect postmolar GTN.
- 7.
GTN is defined by a plateaued, rising, or prolonged persistence of elevated hCG values after molar evacuation; histologic diagnosis of choriocarcinoma, invasive mole, PSTT, or ETT; or identification of metastatic disease after molar evacuation.
- 8.
GTN is staged based on the World Health Organization prognostic score as either low risk (≤6) or high risk (≥7).
- 9.
Low-risk GTN is treated with actinomycin D or methotrexate. Cure rates approach 100%.
- 10.
High risk GTN is treated with EMA/CO (etoposide, methotrexate, actinomycin D, cyclophosphamide, and vincristine) chemotherapy. Cure rates are greater than 90%.
Gestational trophoblastic disease (GTD) comprises a spectrum of neoplastic conditions derived from the placenta. Whereas hydatidiform moles, gestational choriocarcinoma, and placental site trophoblastic tumor (PSTT) are histologic diagnoses, postmolar GTN is defined by clinical and laboratory criteria. The disease entities included in GTD have a wide variation in behavior, but GTN specifically refers to those with the potential for tissue invasion and metastases.
Gestational trophoblastic disease has been recognized since antiquity. Individual hydropic molar villi ( Fig. 7.1 ) were sometimes identified as separate fetuses. In the early 19th century, Velpeau and Boivin recognized hydatidiform mole as a cystic dilation of the chorionic villi. In 1895, Marchand demonstrated that hydatidiform mole and less frequently normal pregnancy preceded the development of choriocarcinoma. In the early 20th century, Fels identified elevated chorionic gonadotropic hormones in the urine of women with hydatidiform moles.
Before the mid-1950s, the prognosis for women with GTN was dismal, and there were no effective treatments. In the 1940s, Hertz demonstrated that fetal tissues required large amounts of folic acid and that their growth could be inhibited by methotrexate. However, it was not until 1956 that Li reported the first sustained remission in a patient with choriocarcinoma treated with methotrexate.
Since that report, GTN has been recognized as the most curable gynecologic malignancy. Reasons for this include (1) identification and development of quantitative assays for human chorionic gonadotropin (hCG) in serum and urine allowed hCG to become the prototype for tumor markers; (2) sensitivity of GTN to various chemotherapeutic agents; and (3) identification of risk factors allowing individualization of treatment, often using multimodality treatment with chemotherapy combined with surgery or radiation therapy (or both) in selected cases.
In general, GTD represents a derangement in the development of the conceptus, associated with unregulated trophoblastic proliferation and invasion, with the propensity for hematogenous metastasis in GTN. The diseases are characterized by paraneoplastic disorders from secretion of gestational hormones, most notably hCG. GTD is the only group of female reproductive neoplasms derived from paternal genetic material (androgenic origin).
Although largely unclear, the etiology of GTD likely involves genetic abnormalities involved in fertilization. Abnormalities in differentiation and pronuclear cleavage, decidual implantation, myometrial invasion, and host immunologic tolerance are also involved. Several candidate tumor suppressor genes (eg, DOC-2/hDab2), chromosomal loci (e.g., 7qp12-7q11,23 and 9q13,3-13,4), and oncogenes (eg, CD9) have been implicated in the pathogenesis of GTD.
Hydatidiform Mole
Two distinct forms of molar pregnancies, complete (CM) and partial (PM) moles, are currently recognized ( Table 7.1 ). Cytogenetic studies have conclusively demonstrated that they are completely separate but related entities. Despite the cytogenetic, pathologic, and clinical differences outlined in Table 7.1 , the clinical management of patients with CMs and PMs is similar.
| Feature | Partial Mole | Complete Mole |
|---|---|---|
| Karyotype | Most commonly 69,XXX or −,XXY | Most commonly 46,XX or −,XY |
| Pathology | ||
| Fetus | Often present | Absent |
| Amnion, fetal RBC | Usually present | Absent |
| Villous edema | Variable, focal | Diffuse |
| Trophoblastic proliferation | Focal, slight to moderate | Diffuse, slight to severe |
| CLINICAL PRESENTATION | ||
| Diagnosis | Missed abortion | Molar gestation |
| Uterine size | Small for dates | 50% large for dates |
| Theca-lutein cysts | Rare | 25%–30% |
| Medical complications | Rare | 10%–25% |
| Postmolar GTN | 2.5%–7.5% | 6.8%–20% |
Epidemiology
The incidence of molar pregnancies in the United States is approximately 1 in 1500 pregnancies. Race or ethnicity, age, socioeconomic status, diet, and prior reproductive history all influence the risk. The most reliable studies suggest that the incidence of hydatidiform mole is slightly less than 1 in 1000 pregnancies in most of the world, 1.2 in 1000 in South Africa, as high as 2 in 1000 in Japan, 7 in 1000 deliveries in Malaysia and the Philippines, and possibly higher in Saudi Arabia. The reported incidence of molar pregnancies among various ethnicities and races may be biased as a result of dependence on hospital records, particularly from tertiary referral centers, rather than population-based studies. Historically, GTD was thought to occur more frequently in the Asian population; however, recent population-based studies in Japan have shown a decreasing incidence of GTD in Japan, even with a relatively homogeneous population.
Extremes of reproductive age are associated with increased risk of molar pregnancy. Based on an analysis of 2202 patients with hydatidiform moles compared with a contemporary control group including all types of pregnancy events, a Duke study found a significantly higher incidence of molar pregnancy in women younger than 15 years and older than 40 years of age. Other studies have confirmed the peak incidence of molar pregnancies at the extremes of maternal reproductive age. Some of these studies noted an increased risk associated with increasing paternal reproductive age for CM but not for PM.
Dietary factors associated with an increased risk of hydatidiform mole may include a low-protein diet and vitamin A deficiency, although these factors are difficult to separate from effects of lower socioeconomic class alone. Berkowitz and colleagues have suggested that a deficiency of animal fat and fat-soluble vitamin carotene is associated with increased risk of molar pregnancies. Populations with a high prevalence of vitamin A deficiency have been noted to have higher incidences of hydatidiform mole. Although carotene-rich vegetables are available in these countries, the lack of dietary fat needed for carotene absorption may result in an overall carotene-deficient condition.
Prior molar pregnancy also increases the risk of recurrent molar pregnancy. Women with a single previous molar pregnancy have more than 10 times the risk of having another molar pregnancy compared with those who have never had one. Based on data by Bagshawe and colleagues, after having had one molar pregnancy, the risk for a second was 1 in 76; after two molar pregnancies, the risk for a third was 1 in 6.5. Sand reported that the risk of a third molar pregnancy was 28%. Goldstein and colleagues noted that 9 of 1339 patients (1 in 150) had at least two consecutive molar pregnancies. Other centers have reported that the incidence of a second molar pregnancy is as high as 1 in 50 women with one prior molar pregnancy.
With recurrent molar pregnancies, there is also an increased risk of developing malignant GTN, although patients with consecutive molar pregnancies may have subsequent normal pregnancies. Berkowitz and colleagues noted that four of their patients with consecutive molar pregnancies later had full-term pregnancies. Lurain noted that five of eight patients with consecutive molar pregnancies later had normal full-term pregnancies.
In a case-controlled study from Baltimore, factors found to be associated with GTN included professional occupation, history of prior spontaneous abortions, and the mean number of months from antecedent pregnancy to the index pregnancy. Contraceptive history, irradiation, ABO blood groups, and smoking factors of the male partner were not associated with development of GTN. Among PMs, no clinical factor such as gravidity, parity, age, uterine size, gestational age at diagnosis, or hCG levels at presentation were associated with development of GTN.
Cytogenetics and Pathology
Molar pregnancies consist of CMs and PMs. In most series, CM and PM comprise approximately 70% and 30% of molar pregnancies, respectively.
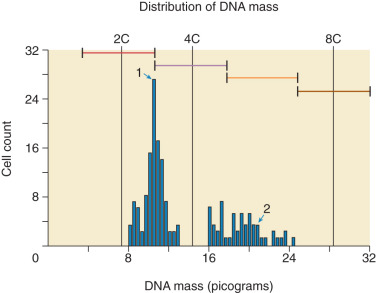
Complete hydatidiform moles are completely derived from the paternal genome (see Table 7.1 ). Most frequently, CMs have a diploid 46, XX genome with exclusively androgenetic markers on the chromosomes, implying duplication of a haploid sperm chromosomal complement in the fertilized ovum. Approximately 5% of CMs have a 46,XY androgenetic genome, indicating that dispermic fertilization occurs in some. The mechanism for exclusion of the maternal polar body from the nucleus of the fertilized ovum is unknown. No 46,YY moles have been reported, but aneuploid karyotypes, such as tetraploidy, have been associated with CM. Fetal red blood cells (RBCs) are not observed in microscopic sections of CM, because the fetus never develops a cardiovascular system.
In contrast, PMs are composed of both paternal and maternal genomes (see Table 7.1 ). Usually, two paternal haploid sets of chromosomes are combined with one maternal set, resulting in complete triploidy ( Fig. 7.2 ). The 69,XXX karyotype is most common, but some PM, have a 69,XXY karyotype, implying dispermic fertilization. Sometimes other aneuploid karyotypes are observed in PM but none with duplication of the Y chromosome. All karyotypes reported for PM feature a maternal haploid set and multiples of the paternal chromosomal complement. Gross or histologic evidence of fetal development such as fetal RBCs is a feature of PM that differentiates it from CM.
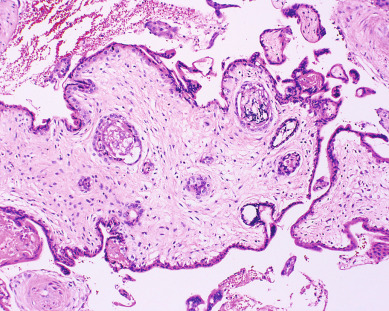
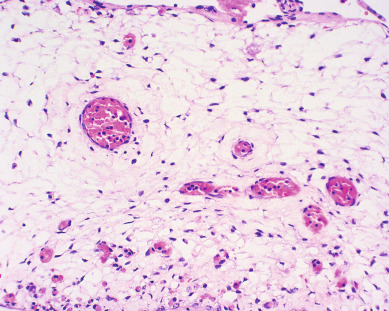
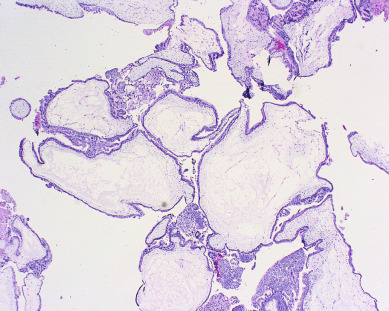
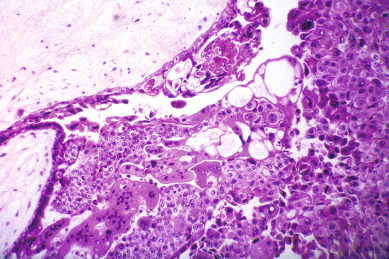
Other histopathologic features of CM and PM differ. CMs have diffuse villous edema, often with central cisterna formation ( Fig. 7.3 ). Trophoblastic proliferation is usually diffuse ( Fig. 7.4 ) but may vary in extent. Histologic evidence of a fetus is lacking. Histologic features of PM can be subtle, and many PMs are probably underdiagnosed erroneously as spontaneous abortions. A PM exhibits focal, varying degrees of hydropic villi with scalloping and trophoblastic inclusions within the villi ( Fig. 7.5 ). Focal trophoblastic proliferation is usually subtle compared with CM. Fetal RBCs are observed within vessels of the villi ( Fig. 7.6 ), and sometimes a fetus can be grossly identified. The fetus is usually nonviable and rarely survives beyond 20 weeks of gestation. The difference in the amount of trophoblastic proliferation between CM and PM is related to their different clinical presentations. Malignant GTN occurs in 2.5% to 7.5% of patients with PM compared with approximately 6.8% to more than 20% after evacuation of a CM mole (see Table 7.1 ).
Other techniques have been developed to differentiate CM from PM. These include karyotyping, flow cytometry, fluorescence in situ hybridization, and amplification of short tandem repeat loci. Additionally, p57 immunohistochemistry is absent in CM but present in PM because it is paternally imprinted but maternally expressed. Although most cases of molar pregnancy can be classified as CM or PM using histopathology alone, histologic differences may not be apparent if the diagnosis is made early in gestation. In this scenario, molecular genetic techniques may be critical for accurate diagnosis.
Presentation and Symptoms
Irregular bleeding is the hallmark symptom of hydatidiform moles. Nearly all patients with moles have delayed or irregular menses for varying time periods. Vaginal bleeding usually occurs during the first trimester. The bleeding may vary from spotting to hemorrhage requiring blood transfusion. In earlier series, bleeding occurred in 73% to 100% of molar pregnancies including CM and PM. Expulsion of recognizable molar vesicles may accompany vaginal bleeding when the gestation approaches the second trimester.
There is increasing information that the presentation of moles may be changing as a result of earlier and improved diagnostic tests, including early ultrasound and hCG serum assays. The New England Trophoblastic Disease Center (NETDC) group compared symptoms in CM treated at their institution from 1994 to 2013 to those treated from 1988 to 1993. Patients in the more recent cohort were less likely to present with vaginal bleeding, 46% versus 84% in the earlier cohort. Patients in the recent cohort were also more likely to be diagnosed with a molar pregnancy at less than 11 weeks (56% vs. 41%). However, earlier diagnosis did not result in decreased rates of GTN.
Other symptoms include nausea and vomiting caused by the high levels of hCG. Nausea and vomiting were reported in almost one-third of patients with hydatidiform mole in older studies. However, in the most recent series from the NETDC, 14% of 180 patients presented with hyperemesis from 1994 to 2013. Curry and colleagues also reported hyperemesis in 14% of patients.
Preeclampsia-like symptoms in the first trimester of pregnancy are nearly pathognomonic of a hydatidiform mole. Symptoms of headache with hypertension, hyperreflexia, and proteinuria define preeclampsia. Preeclampsia was present in 1% of patients in the NETDC cohort and in 12% of the patients in the study by Curry and colleagues. Fortunately, eclampsia in the setting of hydatidiform mole is rare.
Molar pregnancy is also associated with other medical comorbidities such as tachycardia and hypertension from hyperthyroidism or shortness of breath and chest pain from acute respiratory distress syndrome. Hyperthyroidism occurs rarely but can be very severe. Laboratory evidence of hyperthyroidism can occur in as many as 10% of patients; however, clinical manifestations are less frequent. Hyperthyroidism is caused by the production of elevated levels of normal hCG by molar tissue. hCG has structural homology with the thyroid-stimulating hormone (TSH) molecule and binds to TSH receptors, which results in thyroid hyperfunction. Furthermore, Yoshimura and colleagues demonstrated that the isoforms of hCG produced by molar pregnancies have higher thyrotrophic activity compared with the hCG produced by normal pregnancies. Clinical manifestations of hyperthyroidism disappear after the molar pregnancy is evacuated. Antithyroid therapy may be indicated for a short period to control hyperthyroidism during molar evacuation. Specifically, the use of beta-blockers to prevent tachycardia may be indicated.
Acute respiratory distress caused by trophoblastic pulmonary embolization can also occur. It is very rare, occurring in only 2% of patients in the NETDC cohort. These patients often have coexisting pathology that may alter cardiac or pulmonary function such as preeclampsia, hyperthyroidism, and anemia, and this combination can contribute to acute cardiopulmonary decompensation. Respiratory distress is most often associated with a large volume of molar tissue and uterine enlargement greater than 16 weeks’ gestational size.
Theca-lutein cysts are caused by hyperstimulation of the ovaries by excessive hCG production. Traditionally, approximately 15% to 25% of patients with unevacuated molar pregnancies present with theca-lutein cysts larger than 6 cm. More recent series have shown rates of theca-lutein cysts at presentation of 6% to 9%. Although theca-lutein cysts will resolve after molar evacuation, there may be considerable lag behind the decline of hCG levels. Surgical intervention is rarely required for acute torsion or bleeding. Patients who develop theca-lutein cysts have a higher incidence of postmolar GTN. Furthermore, patients with the combination of enlarged ovaries with a large for gestational age uterus results are at a high risk for malignant GTN. Up to 57% of patients with this combination require subsequent therapy for postmolar GTN.
Classically, a patient with a hydatidiform mole was said to have a uterine size excessive for gestational age. Historically, this was found in more than 50% of patients with CM; however, approximately one-third of patients have uteri smaller than expected for gestational age. In the NETDC cohort from 1994 to 2013, uterine size was excessive for dates in only 24%.
Similar to the comparative study performed by NETDC, Mangili and colleagues from Italy compared clinical features of molar pregnancies between two historical cohorts, 1970 to 1982 versus 1992 to 2004. The later cohort had significantly less vaginal bleeding on presentation (51% vs. 74%, P <0.0001), fewer with increased uterine volume (29% vs. 51%, P <0.0001), and fewer with theca-lutein cysts (13% vs. 21%, P = 0.03). There was no difference in rates of hyperemesis or preeclampsia. Because molar pregnancies are diagnosed at an increasingly early gestational age, clinical symptoms are less frequent. As a result, there is increasing reliance on histopathologic analysis to identify cases of molar pregnancies.
The previously outlined conditions are seen mainly in patients with CM. Patients with PM usually do not exhibit excessive uterine size, theca-lutein cysts, preeclampsia, hyperthyroidism, or respiratory problems. In most patients with PM, the clinical and ultrasound diagnosis is usually a missed or incomplete abortion. PMs are often underdiagnosed because of the lack of clinical suspicion and the often subtle or focal nature of the pathologic changes in the placental tissues. Therefore, products of conception from missed or incomplete abortions should be thoroughly examined by pathologists to prevent a missed diagnosis of PM. Cytogenetic testing may be required to accurately diagnose GTD.
Diagnosis
Patients with CM are often clinically diagnosed by characteristic ultrasound features and a markedly elevated hCG. In contrast, the majority of patients with PM and an increasing proportion of patients with CM are clinically misdiagnosed as missed abortion. A quantitative hCG of greater than 100,000 IU/L, an enlarged uterus with absent fetal heart sounds, and vaginal bleeding suggest a diagnosis of hydatidiform mole. However, a single hCG determination is not diagnostic. A single high hCG value may be seen with a normal single or multiple pregnancy, especially if there has been bleeding or disruption of the placenta. Therefore, an isolated hCG value alone should not be used as the sole determining factor in diagnosing hydatidiform mole. Conversely, a “normal” hCG level for an anticipated gestational age does not exclude the possibility of a molar pregnancy.
Ultrasonography has replaced all other radiographic means (eg, amniography or uterine angiography) for diagnosis of hydatidiform mole. Molar tissue typically is identified as a diffuse mixed echogenic and vesicular pattern replacing the placenta ( Fig. 7.7 ), traditionally labeled a “snowstorm appearance.” Recently, with increasing use of early ultrasonography, classic features may be subtle or lacking in cases of early CM or PM. In a recent series based on histopathologic identification of molar pregnancies, 52% of patients were diagnosed based on ultrasound examination before evacuation of the uterus. In another series, the abundant vesicular pattern and “snowstorm appearance” were absent in 10% of CM pregnancies and only subtly present in 63% of CM based on ultrasonography performed at mean gestational age of 7 weeks and 6 days.
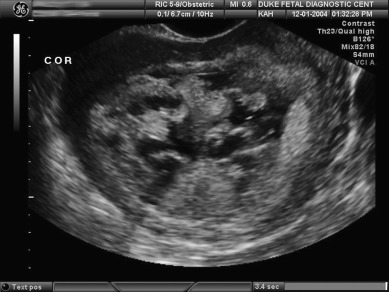
The largest study to date, from Charing Cross Hospital in the United Kingdom, assessed more than 1000 cases of GTD from 2002 to 2005 and found an overall 44% detection rate by ultrasonography of molar pregnancies before evacuation. The overall detection rate was 35% to 40% before 14 weeks of gestation and 60% after 14 weeks. The sensitivity was 44%, and the specificity was 74%. The positive predictive value was 88%, and the negative predictive value was 23% for detecting molar pregnancy of any type. Detection rates were higher for CM versus PM and for later gestations. Nonetheless, fewer than 50% of molar pregnancies were diagnosed by ultrasonography in first-trimester scans.
In a smaller series from St. George’s in London, the sensitivity of ultrasonography for the diagnosis of a CM was 95% and 20% for PM. Another series evaluated the use of both ultrasound and hCG values to diagnose hydatidiform mole. When ultrasonography was used alone, 42% of patients with molar pregnancies did not have a definite diagnosis on first examination. When the hCG value was above a threshold of 82,350 mIU/mL and was used with the initial ultrasound findings, 89% of hydatidiform moles were correctly identified ( Fig. 7.8 ). A combined algorithm using hCG, clinical history, examination, and imaging is required to make the diagnosis of hydatidiform mole.
Several reports have been made of a hydatidiform mole arising in ectopic sites, such as the fallopian tube. These patients present with classic symptoms and signs of ectopic pregnancy, including occasionally with tubal rupture and hemorrhagic shock. Tubal GTD was diagnosed in 16 (0.8%) of 2100 women with GTD who were managed at the NETDC. Various authors have warned that the current trend of treating ectopic pregnancies with conservative surgery or single-dose methotrexate necessitates close monitoring of serum hCG levels to avoid missing the diagnosis of ectopic GTD.
Evacuation
Given the subtle presentation of GTD in the modern era, the diagnosis of molar pregnancy will increasingly be made only after histologic evaluation of uterine curettings. Curettage is often performed for a suspected incomplete spontaneous abortion. When GTD is diagnosed, patients should be monitored with serial hCG measurements. Baseline postevacuation chest radiography should be considered. For patients in whom hydatidiform mole is suspected before evacuation, the following laboratory evaluation is recommended ( Table 7.2 ): complete blood count with platelet determination, clotting function studies, renal and liver function studies, blood type with antibody screen, and hCG level. A pre-evacuation chest radiograph should also be obtained.
| Evacuation: suction D&E (or hysterectomy in selected patients) |
| Postevacuation quantitative hCG level and chest radiography |
| Monitor quantitative hCG levels every 1–2 weeks until three normal values or criteria for GTN |
| After hCG level is normal for three values, then monitor hCG levels every 1–3 months for 6 months |
Initiate chemotherapy for GTN using indications listed in Table 7.4 :
|
Evacuation of molar pregnancies should be performed expeditiously. Medical complications are observed in approximately 25% of patients with molar pregnancy and uterine enlargement of greater than 14 to 16 weeks’ gestational size. The molar pregnancy should be evacuated as soon as possible after stabilization of any medical complications. The choice of facilities for molar evacuation should be based on the expertise of the physician, uterine size, and ability of the facility to manage existing medical complications. In most patients, the preferred method of evacuation is suction dilation and evacuation (D&E) (see Table 7.2 ).
Medical induction of labor with oxytocin or prostaglandin and hysterotomy are not recommended for evacuation because they increase blood loss and may increase the risk for malignant sequelae compared with suction D&E. The Charing Cross group reported a significant trend toward more frequent evacuation by suction curettage compared with sharp curettage or medical induction for molar evacuation over their study interval. Postmolar GTN developed in 5.9%, 3.8%, and 9.1% of their patients evacuated with suction curettage, sharp curettage, and medical induction, respectively ( P <0.05 for increased postmolar GTN in the medical induction group). Furthermore, many patients require D&E to complete the evacuation of the mole after medical induction of labor.
Evacuation is usually performed under general anesthesia, but local or regional anesthesia may be used for a cooperative patient with a small uterus. After serial dilatation of the cervix, uterine evacuation is accomplished with the largest cannula that can be introduced through the cervix. Most providers begin curettage in the lower uterine segment, allowing the uterus to contract progressively as the pregnancy is evacuated. Evacuation may be accompanied by significant blood loss during evacuation of tissue, particularly with increasing gestational age. Intravenous (IV) oxytocin is begun after the cervix is dilated and continued for serval hours postoperatively to assist with uterine contractility. Other uterotonics such as methylergonovine or misoprostol may be used if needed. After completion of suction D&E, gentle sharp curettage may be performed. Repeat uterine evacuation by D&E has been performed for persistent GTD, defined in the United Kingdom as failure of hCG to normalize within 4 to 6 weeks or an increase in hCG any time after primary D&C. The utility of repeat second D&C is controversial. It may subject patients to surgical complications, when hCG may normalize given more time. Additionally, it does not comply with current International Federation of Gynecology and Obstetrics (FIGO) guidelines for treatment of GTN.
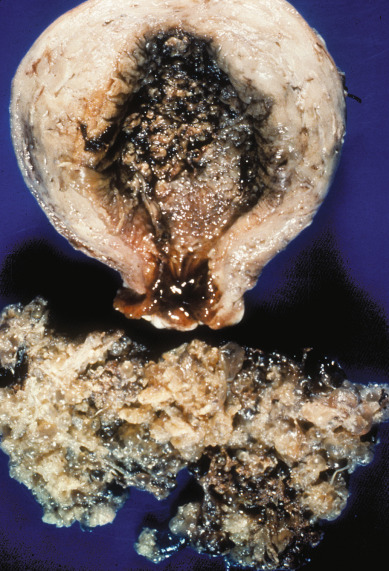
Hysterectomy is an alternative to suction D&E for molar evacuation in selected patients who do not wish to preserve childbearing ( Fig. 7.9 ). The adnexa are routinely preserved, and theca-lutein cysts should be left in situ unless they are torsed or ruptured and actively bleeding. Hysterectomy reduces, but does not eliminate, the risk of malignant postmolar sequelae. The risk of postmolar GTN after hysterectomy remains approximately 3% to 5%; therefore, these patients should be monitored postoperatively with serial hCG levels.
Risk Factors for Postmolar Gestational Trophoblastic Neoplasia
Many of the risk factors for development of postmolar GTN indirectly reflect the amount of trophoblastic proliferation at the time of evacuation. High pre-evacuation hCG levels, uterine size larger than expected by dates, and theca-lutein cysts (see Fig. 7.9 ) increase the risk. Many of these factors interact. The combination of theca-lutein cysts and uterus larger than expected for dates increases the risk of postmolar GTN to 57%. Other series have used multivariate analysis to identify other risk factors for postmolar GTN and have found that age, parity, initial uterine size, presence of theca-lutein cysts, and initial hCG concentration were not independent prognostic factors.
Multiple risk factors have been incorporated into scoring systems that seek to categorize patients and predict which patients will develop postmolar GTN. However, many of these efforts have not been successful. Parazzini and colleagues found that, although 15% of their patients had risk factors that classified them as “high risk,” these patients accounted for a minority of the cases of postmolar GTN. Of note, despite the earlier diagnosis and decreasing symptom burden among patients in the modern era with molar pregnancies, there has not been a decrease in the incidence of postmolar GTN. This implies that other molecular biologic mechanisms may be responsible for the development of postmolar GTN rather than the amount of trophoblastic proliferation at the time of evacuation. Most centers do not treat “high-risk” molar pregnancies with postevacuation chemotherapy to prevent the development of postmolar GTN; rather, they rely on surveillance with serial hCG levels to diagnose postmolar GTN and then treat it when encountered.
Postmolar Surveillance
After molar evacuation, it is important to monitor all patients to diagnose and treat postmolar GTN promptly. Serial quantitative serum hCG measurements should be performed using one of several commercially available assays capable of detecting hCG to baseline values (<5 mIU/mL). Ideally, serum hCG levels should be obtained every 1 to 2 weeks until three consecutive values are normal. hCG can then be measured at 1- to 3-month intervals until 6 months after the initial normal value (see Table 7.2 ). Reliable contraception is highly recommended during monitoring of hCG values. Chest radiography is indicated if the hCG level rises. Patients with a histologic diagnosis of malignant GTN (choriocarcinoma, invasive mole, or PSTT), the development of metastatic disease, a rising hCG value, or a plateauing of the hCG values over several weeks’ time are diagnosed with postmolar GTN, as discussed later. Whereas approximately 15% to 20% of patients develop postmolar GTN after evacuation of a CM, 1% to 5% of patients develop postmolar GTN after a PM.
Postmolar surveillance by serial hCG monitoring after achieving normal values allows identification of patients who develop late GTN. Although rare instances of long latent periods between molar evacuation and postmolar GTN have been reported, the vast majority of episodes of GTN after hydatidiform moles occur within approximately 6 months of evacuation. The NETDC analyzed 1029 patients followed with hCG assays after CM evacuation at their institution. Postmolar GTN occurred in 153 (15%) patients. Only two women developed postmolar GTN after normalization of hCG levels, and both were followed by an older assay with a sensitivity of 10 mIU/mL.
None of their 82 (95% confidence interval [CI], 0%–4.5%) patients who were followed with an hCG assay with a sensitivity of 5 mIU/mL developed postmolar GTN after their hCG values normalized. However, a substantial number of their patients were excluded from analysis because of incomplete follow-up, including 817 patients lost to follow-up after normalization of hCG and 60 patients lost to follow-up before hCG values normalized. The same group of investigators reported that patients with PM had spontaneous normalization of hCG, with a median time of 46 days, and suggested that surveillance in this group may also be abbreviated.
Kerkmeijer and colleagues from the Netherlands reported that 74.6% patients with molar pregnancies attained spontaneous normalization of hCG after evacuation. Only one case of GTN occurred out of 265 patients who obtained two normal hCG levels. They conclude that hCG surveillance can also be curtailed after normalization of values. Within the same database, they found that no GTN occurred in patients with CM who obtained spontaneous normalization of weekly hCG levels within 2 months after evacuation. Because this total experience is relatively small and plagued by incomplete follow-up, it is prudent to recommend continued surveillance after molar evacuation with hCG monitoring for 6 months after normalization of hCG values. However, it should be noted that the risk of postmolar GTN is quite low after normalization.
Although early pregnancies after molar evacuation are usually normal gestations, an early pregnancy obscures detection of an elevated hCG caused by GTN. Oral contraceptives do not increase the incidence of postmolar GTN or alter the pattern of regression of hCG values. Special consideration should be given to oral contraceptive pills as the contraceptive of choice because they suppress the luteinizing hormone (LH) surge by preventing ovulation. LH shares an alpha subunit with hCG, and thus an LH surge may lead to a false-positive hCG value. In a randomized study conducted by the Gynecologic Oncology Group (GOG), patients treated with oral contraceptives had half as many intercurrent pregnancies during hCG surveillance as those using barrier methods. Furthermore, the incidence of postmolar GTN was lower in patients using oral contraceptives.
After completion of surveillance documenting remission for 6 months, pregnancy can be encouraged and hCG monitoring discontinued. Patients with a history of molar pregnancy remain at an elevated risk of GTN after all future pregnancies. Thus, pathologic examination of all future pregnancies is recommended, including placental examination and examination of the products of conception. Patients should also obtain a serum hCG value at 6 weeks after any pregnancy event (delivery or spontaneous or therapeutic abortion).
Prophylactic Chemotherapy After Molar Evacuation
Two randomized studies have evaluated prophylactic chemotherapy after molar evacuation. Kim and colleagues reported that a single course of methotrexate–folinic acid reduced the incidence of postmolar GTN from 47.4% to 14.3% ( P <0.05) in patients with high-risk molar pregnancies, but the incidence was not reduced in patients with low-risk molar pregnancies. Patients who received prophylactic chemotherapy but developed GTN required more chemotherapy than those who had not been exposed to prophylactic chemotherapy. In the study by Limpongsanurak, a single course of actinomycin D was compared with observation in patients after evacuation of high-risk molar pregnancies. Postmolar GTN was 50% in the control group compared with 13.8% in the treatment group ( P <0.05). In both studies there were no deaths in the treatment or control groups caused by GTN or treatment toxicity.
However, there are anecdotal cases of fatalities caused by prophylactic chemotherapy, and prophylactic chemotherapy does not eliminate the need for postevacuation follow-up. Furthermore, patients are exposed to drugs most frequently used to treat postmolar GTN, which could lead to relative chemoresistance. Receipt of chemotherapy is also associated with decreased quality of life, cost, and morbidity. In compliant patients, the low morbidity and mortality achieved by monitoring patients and instituting chemotherapy only in patients with postmolar GTN appears to outweigh the potential risk and small benefit of routine prophylactic chemotherapy. Exceptions to this are scenarios in which hCG follow-up is not possible because of patient or system factors; prophylactic chemotherapy may be considered in these cases.
Coexistent Molar Pregnancy With a Normal Fetus
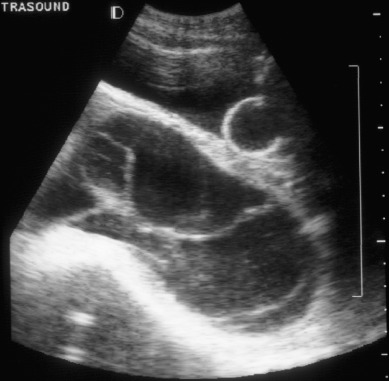
Coexistence of a genetically normal fetus with molar change of the placenta is relatively rare ( Fig. 7.10 ), occurring in 1 in 22,000 to 1 in 100,000 pregnancies. The majority of the literature covering this relatively rare entity consists of case reports and case series. Both CM and PM with a coexistent normal fetus have been reported. A variety of criteria have been used to evaluate these pregnancies. Many of the reports that antedated the histologic and cytogenetic distinction between CM and PM likely included twin gestations with coexistent fetus and molar gestation in addition to singleton PM. Although there might be an increased incidence of coexisting molar pregnancy and fetus related to an increase in multifetal pregnancies caused by ovulation induction for infertility, this may only reflect reporting bias.
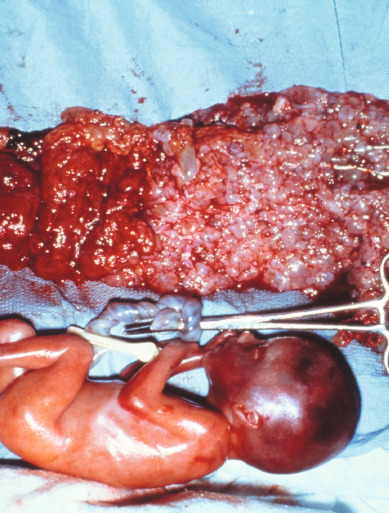
Most of these are diagnosed antepartum by ultrasound findings of a complex, cystic placental component distinct from the fetoplacental unit. However, in a few cases, the diagnosis is not suspected until examination of the placenta after delivery. Medical complications of hydatidiform mole appear to be increased, including hyperthyroidism, hemorrhage, and pregnancy-induced hypertension.
Compared with singleton hydatidiform moles, twin pregnancy with a fetus and mole has an increased risk for postmolar GTN and a higher proportion of patients develop high-risk GTN requiring multiagent chemotherapy. In patients with a coexistent mole and fetus who continue pregnancy beyond 12 weeks, the subset that develop early complications leading to termination of the pregnancy before fetal viability has a markedly increased risk of postmolar GTN compared with patients whose pregnancy continues into the third trimester.
Among 72 patients collected by a national survey of physicians in Japan, 24 patients underwent first-trimester evacuation with 20.8% subsequently developing postmolar GTN. In comparison, 45.2% of 31 patients who required evacuation during the second trimester and 17.6% of the 17 who delivered in the third trimester developed postmolar GTN ( P <0.05). Nine (50%) of the 18 patients with proven androgenic mole in association with a fetus subsequently were treated for postmolar GTN, but this increased risk may have been a result of selection bias. Major congenital abnormalities have not been reported in surviving infants.
For patients with coexistent hydatidiform mole and fetus suspected by ultrasonography, there are no clear guidelines for management. Ultrasonography should be repeated to exclude retroplacental hematoma, other placental abnormalities, and degenerating myoma and to fully evaluate the fetoplacental unit for evidence of a PM or gross fetal malformations. If the diagnosis is still suspected and continuation of pregnancy is desired, fetal karyotype should be obtained, chest radiography should be performed to screen for metastases, and serial serum hCG values should be followed.
Patients are at an increased risk for medical complications of pregnancy requiring evacuation, including bleeding, premature labor, and pregnancy-induced hypertension. They should be counseled about these risks and the increased risk of postmolar GTN after evacuation or second-trimester delivery. If fetal karyotype is normal, major fetal malformations are excluded by ultrasonography, and there is no evidence of metastatic disease, it is reasonable to allow the pregnancy to continue unless pregnancy-related complications force delivery. After delivery, the placenta should be histologically evaluated, and the patient should be followed closely with serial hCG values, as one would with a singleton hydatidiform mole.
Gestational Trophoblastic Neoplasia
GTN is defined by a plateaued, rising, or prolonged persistence of elevated hCG values after molar evacuation; histologic diagnosis of choriocarcinoma, invasive mole, PSTT, epithelioid trophoblastic tumor (ETT); or identification of metastatic disease after molar evacuation. Prompt diagnosis of malignant GTN is important because a delay in diagnosis may increase the patient’s risk and decrease response to treatment. All patients with malignant GTN should undergo a complete evaluation aimed at identifying metastatic sites ( Table 7.3 ) and other clinically important prognostic factors. Several classification systems have been used to determine prognostic groups and assist in the triage of management for individual patients. The FIGO revised its staging system for patients with malignant GTN in 2000 to reflect prognostic factors other than simple anatomic distribution of disease.
| Metastatic Site | Number | (%) | (% Metastatic) |
|---|---|---|---|
| Nonmetastatic | 195 | 54 | |
| Metastatic | 136 | 46 | |
| Lung only | 110 | 81 | |
| Vagina only | 7 | 5 | |
| Central nervous system * | 9 | 7 | |
| Gastrointestinal * | 5 | — | 4 |
| Liver * | 2 | — | 1.5 |
| Kidney * | 1 | — | 0.7 |
| Unknown † | 4 | — | 3 |
* Concurrent lung metastases, highest risk site recorded.
† Rising human chorionic gonadotropin after hysterectomy for hydatidiform mole, no identifiable metastases.
Diagnosis
Between half and two-thirds of cases of GTN that require treatment follow evacuation of CM or PM. Because these patients are treated on the basis of hCG levels rather than hysterectomy, the distribution of histologic lesions is not known. Whereas approximately 50% to 70% of patients with postmolar GTN have persistent or invasive moles, 30% to 50% have postmolar gestational choriocarcinoma.
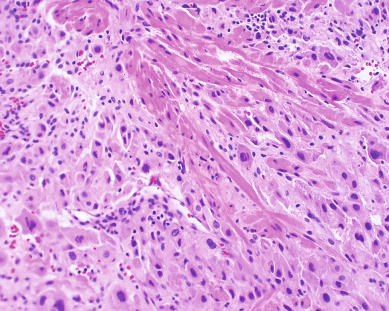
Gestational choriocarcinoma ( Fig. 7.11 ) derived from term pregnancies, spontaneous abortions, and ectopic pregnancies accounts for the vast majority of the other cases of malignant GTN. PSTT ( Fig. 7.12 ) and ETT are rare forms of GTN that can follow any pregnancy event.
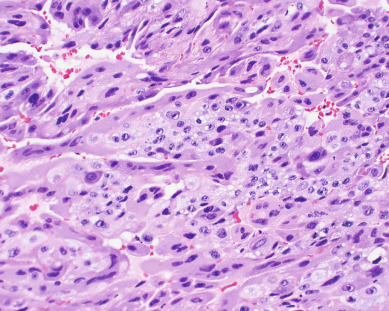
Invasive moles are characterized by the presence of edematous chorionic villi with trophoblastic proliferation that invades directly into the myometrium. Metastasis of molar vesicles may occur. Usually invasive moles undergo spontaneous resolution after many months, but they are treated with chemotherapy to prevent morbidity and mortality caused by uterine perforation, hemorrhage, or infection. Gestational choriocarcinoma is a pure epithelial malignancy, comprising both neoplastic syncytiotrophoblast and cytotrophoblast elements without chorionic villi (see Fig. 7.11 ). Gestational choriocarcinomas are highly malignant, and chemotherapy is indicated.
Placental site trophoblastic tumors are rare. These tumors are characterized by absence of villi with proliferation of intermediate trophoblast cells (see Fig. 7.12 ). The syncytiotrophoblast population observed in choriocarcinoma is lacking in PSTT, with relatively lower levels of intact hCG secreted by these tumors. In general, PSTTs are not as sensitive to chemotherapy. Fortunately, most patients present with disease confined to the uterus and can be treated with hysterectomy.
Women with postmolar GTN are usually diagnosed early in the course of disease by serial hCG monitoring. In contrast, patients with malignant GTN after nonmolar gestations traditionally presented with predominantly nongynecologic symptoms, including hemoptysis or pulmonary embolism, cerebral hemorrhage, gastrointestinal (GI) or urologic hemorrhage, or metastatic malignancy of an unknown primary site. The index pregnancy event may have occurred years before presentation or may have been a subclinical spontaneous abortion. In a series from the NETDC, the presentation of choriocarcinoma after term gestation in the modern era (1996–2011) was compared with an earlier period (1964–1996). They found 30% of women in the modern era were asymptomatic and diagnosed by an incidental positive pregnancy test result or placental pathology compared with 2% in the previous time period ( P = 0.001). The possibility of malignant GTN should be suspected in any woman of reproductive age who presents with metastatic disease from an unknown primary site or cerebral hemorrhage. Under these circumstances, the diagnosis is facilitated by a high index of suspicion that, coupled with serum hCG testing and exclusion of a concurrent pregnancy, confirms the diagnosis without need for tissue biopsy.
Before the development of effective chemotherapy, 10% of patients required hysterectomy after molar evacuation for the treatment of invasive molar pregnancy or choriocarcinoma. Other prechemotherapy reports indicated a mortality rate of more than 20% among patients with invasive mole. Since the development of chemotherapy, between 15% and 36% of patients in the United States are treated after molar evacuation based on inclusive hCG level criteria for initiating chemotherapy in an attempt to limit the morbidity and mortality caused by postmolar GTN. In contrast, Bagshawe and colleagues used very conservative hCG criteria to initiate chemotherapy and reported treating only approximately 8% of their patients after molar evacuation.
The pattern of hCG regression after evacuation of hydatidiform mole is most often used to make a diagnosis of postmolar GTN. After evacuation, most patients have an initial decrease in hCG levels and should be followed with serial hCG values every 1 to 2 weeks. Almost every series has treated patients on the basis of a confirmed hCG rise. In many studies, however, chemotherapy was initiated on the basis of an hCG level plateau of relatively short (<3 weeks) duration. Kohorn noted that 15% of his patients had hCG plateaus after molar evacuation that lasted at least 2 weeks followed by spontaneous regression without intervention. In a trial of actinomycin D, Schlaerth and colleagues observed that 25% of their patients had a substantial spontaneous decrease in hCG levels on the day that chemotherapy was initiated. Based on the correlation between hCG level and tumor burden, Kohorn has suggested an approach using shorter observation periods for patients with high hCG level plateaus but allowing longer periods of observation for those who have hCG plateaus at levels under 1000 mIU/mL.
In some studies, an additional criterion for initiating chemotherapy after molar evacuation has been persistence of hCG for an arbitrary length of time, ranging between 6 weeks and 6 months after evacuation. The initial reports of chemotherapy from the National Institutes of Health (NIH) used persistence of hCG for more than 60 days after molar evacuation as an indication for chemotherapy treatment. Many series have documented that persistence of hCG for more than 60 days after evacuation increases the risk for developing postmolar GTN. However, 60% to 64% of patients with delayed regression of hCG beyond 60 days will ultimately undergo spontaneous hCG regression, with no deaths in those who began therapy more than 60 days from molar evacuation. Furthermore, groups reporting from the United Kingdom, where care for GTD patients is centralized, follow patients with elevated hCG levels for up to 6 months without an increase in morbidity or mortality among those treated after this prolonged follow-up.
Lurain and colleagues from the John I. Brewer Trophoblastic Disease Center reported their experience with 738 patients with complete hydatidiform moles. In their study, 80.7% of patients had spontaneous regression of hCG values to normal, although only 65% had done so by 60 days. An additional 27.9% reached normal values during the next 110 days. Overall, 142 (19%) were treated with chemotherapy because of rising or plateauing hCG levels. Of these, 125 had invasive moles, and 17 had choriocarcinomas. Only 15% of the 142 treated patients, or 3% of the total, developed metastases outside of the uterus. Morrow and Kohorn treated a higher proportion of their patients (36% and 27%, respectively) using hCG criteria derived from regression curves compared with studies that use the individual patient’s hCG regression pattern. It appears that use of hCG regression curves may result in treatment of more patients than necessary, and it did not prevent metastatic disease.
In an effort to provide uniformity to the diagnosis of postmolar GTN, the FIGO conducted workshops among members of the Society of Gynecologic Oncologists, International Society for the Study of Trophoblastic Disease, and International Gynecologic Cancer Society to formulate its current recommendations for evaluation and staging this disease. The current FIGO requirements for making a diagnosis of postmolar GTN are as follows ( Table 7.4 ):
- 1.
Four values or more of plateaued hCG (±10%) over at least 3 weeks: days 1, 7, 14, and 21
- 2.
A rise of hCG of 10% or greater for 3 values or more over at least 2 weeks: days 1, 7, and 14
- 3.
Histologic diagnosis of choriocarcinoma
- 4.
Persistence of hCG beyond 6 months after mole evacuation
| Diagnosis of GTN |
| After molar evacuation: four values or more of plateaued hCG (±10%) over at least 3 weeks: days 1, 7, 14, and 21 |
| After molar evacuation: a rise of hCG of 10% or greater for three values or more over at least 2 weeks: days 1, 7, and 14 |
| After molar evacuation: persistence of hCG beyond 6 months |
| The histologic diagnosis of choriocarcinoma, invasive mole, or PSTT |
| Metastatic disease without established primary site with elevated hCG (pregnancy has been excluded) |
| Evaluation of GTN |
| Complete physical and pelvic examination; baseline hematologic, renal, and hepatic functions |
| Baseline quantitative hCG level |
| Chest radiograph or CT scan of chest |
| Brain MRI |
| CT or MRI scan of abdomen and pelvis |
The level and duration for observation of the hCG plateau beyond 3 weeks would be determined at the discretion of the treating physician. Observation of hCG level plateau for longer than 3 weeks was permitted because this does not appear to have an adverse effect on survival.
Recently, laboratory assays for hyperglycosylated hCG have been found to be highly sensitive for differentiating active GTN or choriocarcinoma from inactive or “quiescent” GTD in patients with hCG level plateaus. These newer assays, developed by Cole and associates at the US hCG Reference Service at the University of New Mexico, may allow earlier diagnosis of postmolar GTN in the future.
The majority of centers in the United States treat patients if metastases are identified. In contrast, Bagshawe and colleagues in the United Kingdom followed patients with pulmonary nodules conservatively and based treatment decisions on hCG regression patterns. Although the overall results from this policy were excellent, outcome for patients with pulmonary metastases was not detailed. In the United States, where care is decentralized, identification of metastases is included as an indication for initiation of therapy.
“Phantom” Human Chorionic Gonadotropin
Rarely, women present with persistently elevated hCG levels but are subsequently found to have a false-positive hCG assay result, sometimes after receiving chemotherapy or surgery for presumed malignant GTN. Most patients with “phantom” hCG present with low-level hCG elevations, but occasionally values as high as 200 to 300 mIU/mL have been recorded. The false-positive hCG values result from nonspecific heterophile antibodies that interfere with the hCG immunometric sandwich assays. Many of these patients have an undefined previous pregnancy event and do not have radiographic evidence of metastatic disease. Serial hCG values usually do not substantially vary, despite prolonged observation, and do not substantially change with therapeutic interventions such as surgery or chemotherapy.
“Phantom” hCG may also present after evacuation of a hydatidiform mole or after a clearly defined pregnancy event, such as an ectopic pregnancy. It should be suspected if hCG values plateau at low levels and do not respond to therapeutic maneuvers such as methotrexate. Evaluation should include study of serum hCG using a variety of assay techniques at different dilutions of patient serum combined with a urinary hCG level if the serum level is above threshold for the urinary assay. Heterophile antibodies are not excreted in the urine. Therefore, if they are the cause of serum hCG level elevation, urinary hCG values will not be detectable. False-positive hCG assays are also not affected by serial dilution of patient sera and have markedly different values using different assay techniques, with the majority of assays reflecting undetectable hCG. Other techniques are available to inactivate or strip the patient’s serum of heterophile antibodies, and many current assays now incorporate these techniques to reduce the incidence of false-positive results. It is important to exclude the possibility of “phantom” hCG before subjecting these patients to hysterectomy or chemotherapy for GTN.
The “Hook Effect”
Instead of being a false-positive test result such as a phantom hCG due to heterophile antibodies, the “hook effect” causes a false-negative hCG value. hCG immunoassays rely on a sandwich assay approach. A sandwich assay approach means that the hCG molecule binds to antibodies attached to a matrix and then additional free-floating “reporter” antibodies bind to the hCG attached to the matrix, and this sandwich of both antibodies on the same molecule is how the hCG level is measured. In the “hook effect,” the assay is overwhelmed by very high levels of hCG, generally greater than 1,000,000 mIU/mL. The large number of hCG molecules bind not only to the antibodies on the matrix but to the reporter antibodies as well. Many fewer or no “sandwiches” are created, and the hCG result will either be falsely low or falsely negative. The “hook effect” can be overcome by serial dilution of the serum before using an hCG immunoassay, and this is standard practice in many laboratories; however, if a false-negative hCG is suspected, serial dilution of the patient’s serum should be performed.
Pretherapy Evaluation
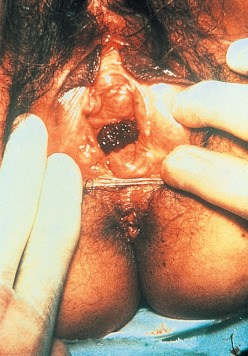
In contrast to epithelial endometrial cancer, GTN usually spreads by hematogenous dissemination. Venous metastasis can occur in the subvaginal venous plexus, resulting in vaginal metastases ( Fig. 7.13 ), or in the main uterine venous system with metastases to the parametrium and lungs. Although direct shunting into the systemic circulation may occur, the majority of disseminated metastases develop only after pulmonary metastases have become established. The brain, liver, GI tract, and kidneys are the distant organs most often affected, but metastases to virtually every organ have been reported. Although lymphatic spread can occur, it is relatively uncommon. The hematogenous pattern of metastatic involvement is important when considering the radiographic evaluation of patients with GTN (see Table 7.3 ).
Pretherapy evaluation of the patient with GTN (see Table 7.4 ) includes assessment of history for clinical risk factors, examination, laboratory evaluation, and radiographic survey for potential sites of metastatic disease and number of lesions. Clinical risk factors important for assigning staging and treatment include duration of disease interval from antecedent pregnancy, type of antecedent pregnancy, and previous treatment. Because of different clinical characteristics, patients with histologically proven ETT and PSTT are classified separately from patients with gestational choriocarcinoma or postmolar GTN.
The laboratory evaluation should include a pretherapy hCG value. It should be emphasized that the hCG level obtained immediately before instituting treatment for malignant GTN is the one used for staging—not the hCG level obtained at the time of molar evacuation.
Vaginal metastases of GTN are diagnosed by physical examination. These lesions usually involve the mucosa of the anterior vagina and present as dark, often blue, soft nodules (see Fig. 7.13 ). Ulceration or active bleeding may be present. Because of the highly vascular nature of these lesions, biopsy is not recommended. If the vagina is the only site of metastasis, the majority of these lesions respond to chemotherapy. A few patients require vaginal packing or selective embolization using interventional radiology to control active hemorrhage.
The recommended radiographic staging evaluation for patients with GTN includes evaluation of the abdomen and pelvis with computed tomography (CT) or magnetic resonance imaging (MRI) and contrasted MRI of the brain. Ultrasonography of the pelvis should also be obtained to exclude the possibility of intrauterine pregnancy before instituting chemotherapy. Both ultrasound and MRI studies of the uterus can be used to identify intrauterine tumors and foci of myometrial invasion by GTN, but they are not sensitive or specific enough to replace serum hCG level monitoring for establishing the diagnosis of GTN or for following patients during treatment.
Approximately 45% of patients have metastatic disease when GTN is diagnosed. As indicated by the anatomic sites of metastatic disease encountered among patients initially treated for malignant GTN (see Table 7.3 ), the lungs are the most frequent site of extrauterine metastasis ( Fig. 7.14 ). Although the majority of patients with high-risk metastatic sites have pulmonary metastases on chest radiograph or symptoms of metastatic involvement, a full metastatic evaluation is recommended for all patients rather than relying on a negative chest radiograph alone to make a determination of nonmetastatic GTN.
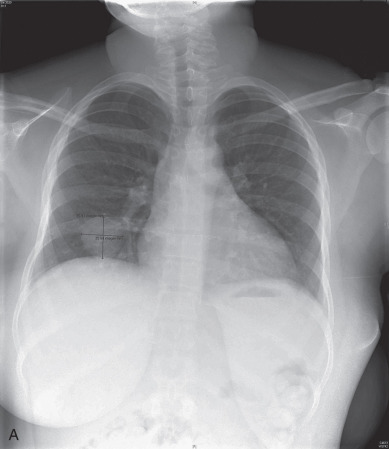
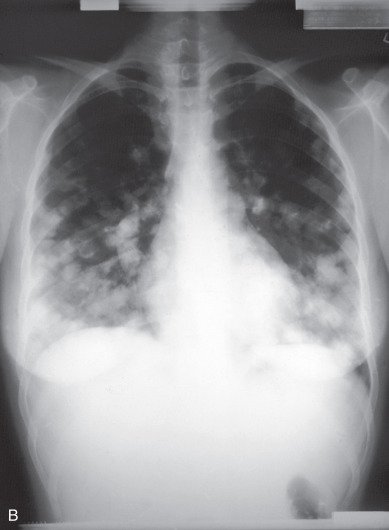
Between 29% and 41% of patients treated for nonmetastatic GTN have pulmonary metastases identified on chest CT that are not detected by chest radiography. The prognostic importance of occult pulmonary metastases is debated when they are the only sites of extrauterine metastases. However, many series of patients treated for high-risk metastases include patients who initially presented with normal chest radiographs. It would be a tragedy to miss the diagnosis of a high-risk metastasis in a patient and delay appropriate aggressive initial therapy.
Although operative procedures may be integrated into the treatment of patients with malignant GTN, they are rarely indicated for establishing the diagnosis or staging. Performing a secondary D&C before beginning chemotherapy in a patient with postmolar GTN is controversial. The experience at the University of Southern California indicated that it had an effect on the treatment in only 20% of patients and was complicated by uterine perforation requiring hysterectomy in 8% of patients. In contrast, Pezeshki and associates analyzed 544 women undergoing a second uterine evacuation for presumed postmolar GTN. After a second evacuation, 368 (68%) entered spontaneous remission. Of 251 patients with a histologic diagnosis of persistent GTD, 96 (38%) required chemotherapy compared with 39 (18%) of 219 with negative histology at the second D&C. A GOG study attempted to answer this question definitively. They prospectively enrolled 64 low-risk nonmetastatic GTN patients and performed a second curettage as initial management of GTN. Only preliminary results are currently available. The authors found that 38% underwent a surgical cure and did not need additional treatment, but 55% underwent progression. They observed one uterine perforation and one grade 3 hemorrhage. Four patients underwent transformation to a PSTT, which would have not been discovered without repeat curettage. The results of this study indicate that a surgical cure is possible but less likely than progression of disease and thus will likely not alter current practice patterns regarding repeat curettage. Many clinicians in the United States treat patients with postmolar GTN without performing a second D&C because of the concern for preservation of fertility. Other operative procedures such as laparoscopy, thoracotomy, or craniotomy are rarely justifiable to establish the primary diagnosis of GTN.
Classification and Staging
The classification and staging of malignant GTN has evolved over the past 50 years. The Clinical Classification System, based on risk factors, was frequently used in the United States ( Table 7.5 ). In this system, patients with nonmetastatic disease are not assigned into a prognostic group because of uniformly good outcome using simple single-agent chemotherapy. The risk factors used in this system were derived by retrospective analysis of resistance to single-agent methotrexate and actinomycin D regimens among women treated for metastatic GTN at the NIH. Hammond and colleagues subsequently reported essentially 100% survival among women who presented with good-prognosis metastatic GTN treated with single-agent therapy. He noted a survival rate of 70% among patients with metastatic poor prognosis disease initially treated with multiagent chemotherapy compared with only 14% survival for patients in this category who initially received single-agent therapy. Poor tolerance of multiagent therapy was noted in the group of poor-prognosis patients receiving initial single-agent regimens, with toxicity causing death in half of these patients.
| Category | Criteria |
|---|---|
| Nonmetastatic GTN | No evidence of metastases; not assigned to prognostic category |
| Metastatic GTN | Any extrauterine metastases |
| Good-prognosis metastatic GTN | Short duration (<4 months) |
| No brain or liver metastases | |
| Pretherapy hCG <40,000 mIU/mL | |
| No antecedent term pregnancy | |
| No prior chemotherapy | |
| Poor-prognosis metastatic GTN | Any one risk factor: Long duration (>4 months) |
| Pretherapy hCG >40,000 mIU/mL | |
| Brain or liver metastases | |
| Antecedent term pregnancy | |
| Prior chemotherapy |
A staging system was adopted by the World Health Organization (WHO) in the early 1980s based on the experience at the Charing Cross Hospital in London between 1957 and 1973. Bivariate analysis was used to identify prognostic factors among patients treated with predominantly single-agent chemotherapy regimens. In the original study, patient age, antecedent pregnancy, interval from antecedent pregnancy, hCG level, ABO blood type, size of the largest tumor, sites and number of metastases, and type of prior chemotherapy were each found to correlate with prognosis. The prognostic index applied a weighted score to each of these factors, which were assumed to act independently. The sum of component scores was then used to determine the individual patient’s risk category.
Multivariate analyses have not confirmed that the factors have independent effects on prognosis. Paternal blood-type information is not uniformly available and was omitted in generating WHO score values by many investigators. Others modified the weighted scoring system using a highest risk score of 6 for each prognostic factor while not changing the total score for assigning patients into risk categories. Furthermore, the WHO prognostic index score provided for reassignment of a score among patients who have previously received treatment for GTN, which might obscure the prognostic effects of some of the other clinical factors on primary treatment. Finally, there was no uniformity in assessing size of the largest tumor or assigning number of metastases. Despite these limitations, modifications of the WHO prognostic index score were useful for predicting risk groups of patients with malignant GTN.
In Table 7.6 , the Clinical Classification System and WHO prognostic index score are compared among patients who presented for initial treatment of GTN at Duke University Medical Center before 1990. The Clinical Classification System had a greater sensitivity for identifying patients at risk for treatment failure and death (see Table 7.6 ). Although the WHO prognostic index score was able to provide slightly better discrimination of patients with poor prognosis metastatic disease in low-, intermediate-, and high-risk populations, stratification into three categories was considered less important than identifying patients at risk of failure for initial therapy. Using multivariate analysis, both systems were roughly equivalent for stratifying patients into risk groups.
| Clinical Classification | Low Risk (<5) | Medium Risk (5–7) | High Risk (>8) | Total Clinical Classification |
|---|---|---|---|---|
| Nonmetastatic GTN | 99.5% (217) | 100% (10) | — | 99.7% (227) |
| Metastatic GTN | ||||
| Good prognosis | 100% (86) | 100% (5) | — | 100% (91) |
| Poor prognosis | 100% (8) | 90.5% (21) | 68.4% (38) | 79.1% (67) |
| Total WHO | 99.8% (311) | 94.4% (36) | 68.4% (38) |
* Data presented as life table survival percentage (number of patients).
In the early 1980s, the FIGO proposed an anatomic staging system for malignant GTN ( Table 7.7 ). Although this system recognized the stepwise development of metastatic disease, it did not incorporate any of the other prognostic factors into the FIGO stage. Although stage I patients uniformly had low-risk disease and stage IV patients had uniformly high-risk disease, there was considerable overlap of outcome within stages II and III. Revision of the FIGO staging in 1992 recognized hCG level and time from antecedent pregnancy as risk factors. These factors generated substages within each stage. Although this revised system did correlate with outcome, it resulted in a proliferation of substages, was confusing for clinicians, and is therefore of questionable importance.