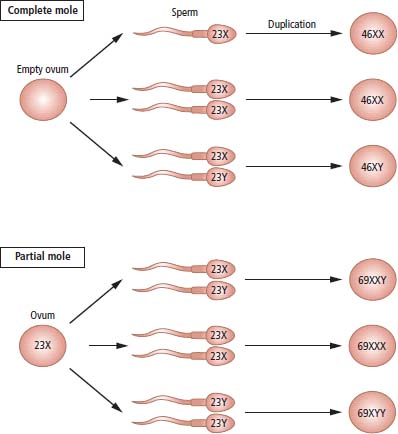
16 Gestational trophoblastic tumours originate from placental tissues and are among the few human cancers that can be cured, even in the presence of widespread metastasis. To a large extent the tumours are the father’s fault as in most cases they contain chiefly paternal DNA. The term gestational trophoblastic disease (GTD) covers hydatidiform molar pregnancies, invasive moles, choriocarcinomas and placental site trophoblastic tumours. Half of the women with choriocarcinoma develop GTD after a molar pregnancy; the remainder have previously had non-molar gestations. Since GTD is relatively rare but can be treated with very high cure rates, all patients should be referred to specialist national units. The incidence of GTD varies geographically, with the highest rates reported from Asia and the risk rises with maternal age and a history of prior molar pregnancy. In the United Kingdom, hydatidiform moles account for one in 1000 pregnancies and about 10% of these will progress to persistent trophoblastic disease requiring chemotherapy. Cytogenetic and molecular analysis of hydatidiform moles has provided a clue as to their origin (Figure 16.1). The majority of complete moles have a 46XX karyotype, with both X-chromosomes of paternal origin (androgenetic). They are believed to originate from fertilization of an empty ovum by a haploid sperm that then underwent duplication. In contrast, partial moles contain both maternal and paternal DNA and are typically triploid 69XXY, presumably as a result of fertilization of a single ovum by two sperm. It is thought that the developmental abnormality affecting uniparental diploid cells in complete moles (in this case androgenetic, 46XX) is due to genomic imprinting. The expression of some genes is determined by their parental origin – whether the allele was inherited from the mother or father – and this persists through multiple rounds of DNA amplification. This parent-of-origin effect is known as genomic imprinting and only affects a minority of genes. Genomic imprinting is an epigenetic phenomenon that does not rely on changes to the DNA base sequence but rather on methylation of individual bases. One example of imprinting in humans is the insulin-like growth factor 2 (IGF2) gene. Only the paternal copy of IGF2 is expressed in foetal life; the silenced maternal gene is said to be “imprinted”. The relaxation of this maternal imprinting results in congenital Beckwith–Wiedemann syndrome: gigantism, macroglossia, exophthalmos, neonatal hypoglycaemia and predisposition to childhood cancers, including Wilms’ tumour, rhabdomyosarcoma and adrenal tumours. Imprinting is also responsible for paired congenital syndromes on chromosome 15q11. This region is differently imprinted in maternal and paternal chromosomes, and both imprintings are needed for normal development. In a normal individual, the maternal allele is methylated, while the paternal allele is unmethylated. Some individuals fail to inherit a properly imprinted 15q11 from one parent, either due to deletion of the 15q11 region from that parent’s chromosome 15 or rarely due to uniparental disomy (in which both copies have been inherited from the one parent). If neither copy of 15q11 has paternal imprinting, the result is Prader–Willi syndrome (characterized by hypotonia, obesity and hypogonadism). If neither copy has maternal imprinting, the result is the Angelman syndrome (characterized by epilepsy, tremors and a perpetually smiling facial expression). Figure 16.1 Possible chromosomal origin of complete and partial hydatidiform moles. Women with trophoblastic disease usually present with antepartum haemorrhage, passing grape-like particles during early pregnancy, anaemia and hyperemesis. Hyperthyroidism may occur because human chorionic gonadotrophin (HCG) acts as a weak thyroid-stimulating hormone (TSH) receptor agonist, due to homology between β-subunits of HCG and TSH, which has been called molecular mimicry. Metastases are typically haemorrhagic. The most frequent sites are the lungs and brain, where they mimic pulmonary thromboembolic disease and subarachnoid haemorrhage. It is therefore always worthwhile performing a pregnancy test in women with these presentations, since normal serum or urine HCG levels exclude this diagnosis. Choriocarcinoma, although rare, is an important diagnosis, as the tumour is exquisitely sensitive to chemotherapy and over 95% of women with this diagnosis can be cured. The definitive diagnostic investigations are a quantitative serum HCG assay and pelvic ultrasonography with colour Doppler flow measurement. Most cases of choriocarcinoma follow a hydatidiform molar pregnancy, although it may also occur after either spontaneous abortion or normal-term pregnancy. If choriocarcinoma follows a molar pregnancy, molecular analysis reveals that the tumour DNA is entirely androgenetic, being derived from the father, with the loss of all maternal alleles. In contrast, post-term choriocarcinoma has a biparental genotype, with DNA from both parents. Nonetheless, all cases of choriocarcinoma include paternal DNA sequences that are absent from the patient’s genome and this may be used to confirm the diagnosis genetically if necessary. GTD was the first cancer to be cured by chemotherapy alone in the early 1960s. A scoring scheme has been devised that determines the risk of developing drug resistance to methotrexate and women at low risk can be successfully treated with single-agent methotrexate, with very high success rates and very few long-term sequelae. Women at higher risk of resistance require combination chemotherapy schedules, which, although still highly successful, run a small risk of causing a second malignancy. Serum HCG acts as the ideal tumour marker in this disease. HCG can be used: The prognosis of gestational trophoblastic tumours is excellent, with the rare exceptions of placental site histological subtype. The cure rates exceed 95%, and much of the current focus of clinical research is aimed at minimizing the long-term side effects of any treatment rather than attempts to increase the cure rates. Case Study: The good casualty officer.
Gestational trophoblastic disease
Epidemiology
Pathogenesis
Presentation
Treatment
Prognosis
 ONLINE RESOURCE
ONLINE RESOURCE
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


