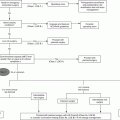
Alzheimer’s
Lewy body
Frontal lobe
Vascular
% of cases
50
20
<10
20
Pathology
Neurofibrillary tangles
Lewy bodies in cortex and amygdala
Degeneration of frontal regions
Varied vascular
Distinct Features
Progressive memory loss
Parkinson-like symptoms; hallucinations; transient loss of consciousness
Behavioral and personality changes
Varied depending on location
Specific medical treatment
Cholinesterase inhibitors and memantine
No specific treatment; cholinesterase inhibitors for cognitive symptoms; Parkinson’s meds for motor symptoms
No specific treatment; Alzheimer’s meds may worsen symptoms
No specific treatment
Alzheimer’s disease is the most common type of dementia with up to 50–70 % of cases. Vascular dementia can have a number of different etiologies and is the second most common behind Alzheimer’s disease. Frontal lobe dementia and Lewy body dementia will also be discussed here.
Alzheimer’s Disease
Alzheimer’s disease (AD) is progressive and irreversible and eventually leads to death [9]. At least 5.4 million people in the United States are affected. It is the sixth leading cause of death in adults. One in eight adults age 65 and over and half of those 85 and over have the disease. Direct costs for caring for patients with Alzheimer’s disease is $200 billion dollars, of which $140 billion is paid by Medicare and Medicaid. By the year 2050, over $1 trillion dollars will be spent on Alzheimer’s disease [10].
Neurofibrillary tangles and neuritic plaques are the characteristic lesions in the brain with Alzheimer’s disease. Beta-amyloid protein is present in these lesions and may play a central role in the disease as may a deficit of acetylcholine and cholinergic areas [11, 12]. A history of head trauma as well as vascular disease increases the risk of Alzheimer’s disease. Clinical features include progressive memory loss, impairment of language, visuospatial ability, and executive function. As in most dementias, behavioral and psychotic symptoms may occur.
Lewy Body Dementia
Lewy body dementia is characterized by Parkinson-like symptoms, recurrent visual hallucinations, neuroleptic sensitivity, fluctuating cognition, falls or syncope, and a transient loss of consciousness. Lewy body dementia may account for up to 20 % of dementia in the United States, affecting up to 1.3 million people. Only 30–50 % of cases are accurately diagnosed [13].
Hallmark lesions are protein deposits known as Lewy bodies. These deposits are located in the cortex and amygdala in Lewy body dementia, as opposed to Parkinson’s, where the deposits are in the brainstem and substantia nigra.
Both the cholinergic and dopaminergic systems are severely disrupted in this disease. Other causes for cognitive decline in the setting of Parkinsonism should be excluded with testing.
Frontal Lobe Dementias
Frontotemporal dementias are a heterogeneous group of disorders that involve degeneration of different regions of the frontal and temporal lobes. Behavioral and personality changes may predominate over cognitive deficits in the clinical picture. These include a loss of personal or social awareness, a lack of insight, inappropriate and stereotyped behaviors, aggression, distraction, a loss of inhibitions, apathy, or extroverted behavior. Some cases involve language and aphasia as primary characteristics. The majority of cases of this group of disorders occur in those under 65 years old.
As this is a group of disorders, the pathophysiology is not well understood.
The cognitive, neuropsychiatric, and behavioral symptoms depend on the regions of the brain involved. Though beginning as a regional process, as the disease progresses, the atrophy and pathology become more generalized.
Vascular Dementia
The term vascular dementia applies to many vascular causes of dementia, including multi-infarct dementia and small vessel disease. About one-fifth of all cases of dementia are vascular. Many of the same things that put one at risk for cardiovascular disease also put one at risk for vascular dementia. These include diabetes, hypercholesterolemia, hyperhomocysteinemia, hypertension, cigarette smoking, and physical inactivity. The effects of these risk factors may vary depending on the type of vascular dementia. Some types are related to specific gene mutations but are rare.
The presenting symptoms and signs will depend on the location of the lesions. Left hemisphere lesions usually cause language problems, and right hemisphere lesions generally cause visuospatial problems. The course may be stepwise, either with abrupt declines or more insidious. Memory or mood complaints are common in both vascular and Alzheimer’s disease. Recognition memory is often preserved in vascular dementia, not in Alzheimer’s. Isolated psychotic symptoms, apathy, and higher cortical disturbance with intact memory may also be vascular dementia.
Cognitive Screening Tests
Dementia is characterized by an acquired and persistent deficit in cognitive domains that interferes with daily functioning. One must first rule out potentially reversible cognitive deficits due to underlying disorders. Laboratory and imaging tests should be done as indicated. Disorders that can mimic dementia include depression, delirium, anticholinergic medications, and toxic metabolic encephalopathy. We will discuss this further in our final section of this chapter.
There are several standardized screening tests for cognitive impairment. One of the oldest and most well known is the Mini-Mental State Examination (MMSE) [14]. It is a brief, 30-point questionnaire which takes about 10 minutes to complete. It can be used to evaluate a patient at a point in time and then repeated to check response to treatment. Categories assessed are orientation to time and place, registration, attention and calculation, recall, language, repetition, and complex commands. A score of 25 or greater is normal. Scores of 21–24, 10–20, and ≤9 indicate mild, moderate, and severe cognitive impairment, respectively. Adjustments may need to be made to the raw score for educational level and age.
The Mini-Cog is a quick and simple method of screening for cognitive dysfunction [15]. It takes about 3–5 minutes to complete. It consists of three-item recall and clock drawing. Clock drawing was used to clarify scores when memory was intermediate. Recall of none of three items signified dementia. Recall of one or two items signified dementia when accompanied by an abnormal clock drawing test. Recall of all three items was considered normal. When compared to the MMSE, the Mini-Cog had better sensitivity at 99 % and correctly classified the greatest percentage (96 %) of subjects. Its diagnostic value was not influenced by education or language [15].
The Montreal Cognitive Assessment (MoCA) is a one-page 30-point test that takes about 10 minutes to complete [16]. The domains assessed are short-term memory recall, visuospatial abilities, executive functions, attention, concentration and working memory, language, and orientation to time and place. MoCA may be better for mild cognitive impairment and early dementias as well as other neurological disorders that affect younger patients such as Huntington’s and Parkinson’s diseases [17–21].
The Saint Louis University Mental Status Examination (SLUMS) is an alternative to the MMSE. In fact, in a large study comparing the two tests, SLUMS was able to better detect mild neurocognitive disorder [22]. Sensitivity and specificity of the two tests are comparable. The test takes about 10 minutes to administer and includes a clock drawing test.
Overview of Treatments
Since the focus of this chapter is the effects of neuropsychological problems on the geriatric trauma patient, treatments of dementia will be addressed briefly. Practitioners should discuss these with the geriatricians and pharmacists. However, treatment of the symptoms that influence our care of the trauma patient will be discussed in more detail.
Alzheimer’s disease had several FDA-approved drug therapies. The cholinesterase inhibitors and memantine are used to address cognitive symptoms. Patients may survive as long as 20 years with Alzheimer’s disease but many patients succumb in the early or middle stages of the disease.
Lewy body dementia has no FDA-approved treatment. As there are cholinergic losses and relationship with Alzheimer’s disease, cholinesterase inhibitors were found to have a role and have become standard treatment for cognitive symptoms [23]. Low doses of Parkinson’s medications, i.e., levodopa, may help motor symptoms, but caution must be used as higher doses can worsen neuropsychiatric symptoms. Average duration of illness is 5–7 years but with much variability.
Frontal lobe dementias have no specific treatments or cures. Not all cases have the same underlying pathology. Cholinesterase inhibitors and memantine may worsen behavioral and psychological symptoms. Long-term care is necessary as the average duration between onset of illness and death is 7 years.
There are no specific treatments for vascular dementia. Control of vascular risk factors is primary. Behavioral and psychological features are treated as necessary.
Behavioral and psychological symptoms including depression occur in the majority of patient with dementia. Psychological symptoms include delusions, hallucinations, paranoia, anxiety, and apathy. Behavioral symptoms include wandering, aggression, hostility, insomnia, inappropriate eating, and abnormal sexual behaviors [24].
Non-pharmacological strategies should be employed in all acute care facilities and are the first-line therapies for behavioral and psychological symptoms in dementia. These consist of environmental and behavioral interventions such as regularly scheduled routines for meals, sleep, and bathing. Reorientation with a clearly visible clock and calendar is indicated. Caregivers should use clear instructions and make frequent eye contact with patients. Sensory impairments, such as vision and hearing loss, should be minimized. These will be addressed in more depth in the section on delirium.
Pharmacological interventions are variable depending on the type of dementia. When medication is necessary, neuropsychiatric symptoms in many dementias have been treated with antipsychotics. Atypical antipsychotics may be better than typical [25]. Quetiapine has been used for psychosis in Parkinsonian syndromes [26]. However, there are concerns regarding the use of these agents. First-generation antipsychotics produce more extrapyramidal symptoms. The second-generation antipsychotics have had a “black box” warning label added by the US Food and Drug Administration for a small but statistically significant increase in cerebrovascular events and death. The older antipsychotics also carry an increased risk of death [27]. In fact, a recent cohort study looking at over 75,000 elderly nursing home patients using antipsychotics found that haloperidol had a higher risk of dying when compared with risperidone [28]. Quetiapine users had a decreased risk of dying. A dose–response relation was noted with all drugs but quetiapine.
Patients with dementia may have a paradoxical agitation when given benzodiazepines and tricyclic antidepressants have unwanted anticholinergic effects. Mood stabilizers, especially SSRIs, may help neuropsychiatric features of frontotemporal dementias.
Cholinesterase inhibitors have been used for neuropsychiatric symptom treatment of Alzheimer’s disease and vascular dementias since cholinergic deficiency also appears to be involved in their development [29]. However, when used in frontotemporal dementias, they may worsen these symptoms.
Neuropsychiatric symptoms in Lewy body dementias can be challenging to treat medically. Older antipsychotic drugs may cause worsening of symptoms and neuroleptic malignant syndrome in Lewy body dementias. As mentioned previously, newer antipsychotics seem to be more beneficial. Benzodiazepines, anticholinergics, and some antidepressants may cause sedation, motor impairment, or confusion. Medications for Parkinsonian symptoms may also worsen confusion, delusions, and hallucinations in higher doses.
In summary, the bottom line is that first-line therapies for neuropsychiatric and behavioral symptoms should be non-pharmacological and medications should be used judiciously and with caution. As the principle of geriatric pharmacological intervention states, “start low, go slow but go.” But in this case, only go if you have to.
Delirium
Definition and Epidemiology
Delirium is a transient, reversible syndrome of impairment of consciousness, attention, and perception in the setting of a medical condition that is acute and fluctuating. The roots of the word are Latin with the term coined by Celsus and included in his work De Medicina. Taking the term apart, “de” is Latin for “away from” and “lira” is the Latin term for “furrow in a field.” So putting it together, it means “going off track.” [30]
Delirium occurs in 60 % of hospitalized frail-elderly patients [31]. One study found that 89 % of survivors of stupor or coma progressed to delirium [32]. Similarly, in the general surgical population, the incidence of delirium is about 37–46 % and postoperative delirium has been described to occur in 10–60 % of patients [33, 34]. Again, the range in incidence of postoperative delirium depends on the type of surgery and the population studied. For example, the incidence of delirium was found to be 65 % after femoral neck fracture repair [35–37]. Approximately seven out of ten surgical intensive care and trauma intensive care patients experience delirium [38].
There are three subtypes of delirium: hyperactive, hypoactive, and mixed. To be diagnosed with hyperactive delirium, the patient must exhibit three or more of the following: hypervigilance, restlessness, fast and/or loud speech, anger, irritability, combativeness, impatience, uncooperativeness, laughing, swearing, singing, euphoria, easy startling, distractibility, nightmares, persistent thoughts, and wandering. For hypoactive, the most difficult to identify, the patient must exhibit four or more of the following: unawareness, lethargy, decreased alertness, decreased motor activity, staring, sparse and/or slow speech, and apathy [39]. Mixed, which is the most common subtype, has features of both.
Delirium has been shown to increase mortality when other factors are controlled for. Studies have shown that the mortality rate at whatever interval studied and in whatever population was significantly higher. Mortality rates range from 8 to 1 % in hospitalized inpatients, 34–15 % 6-month mortality after ICU stay, and 11–3 % 90-day mortality in med-surg patients [31, 40, 41].
The costs of delirium can be staggering, ranging from $38 billion to $152 billion per year in a study of healthcare costs [42]. Though patients with delirium survived fewer days than those without, they had significantly higher adjusted costs, over 2.5 times the costs of patients without delirium. Costs attributable to delirium were $16,303 to $64,421 per patients.
Causes and Risk Factors
The causes and risk factors are many. Temporal relationship to clinical events is an important clue to cause. For example, exposure to midazolam is an independent and potentially modifiable risk factor for the transitioning to delirium [38]. So delirium arising after administration of midazolam would point to the drug as the cause.
The cause may also be determined by the clinical situation or condition. Potentially life-threatening conditions that cause delirium can be remembered by the mnemonic WHHHHIMPS. They are Wernicke’s disease; hypoxia; hypoglycemia; hypertensive encephalopathy; hyperthermia or hypothermia; intracerebral hemorrhage; meningitis/encephalitis; poisoning, either exogenous or iatrogenic; and status epilepticus.
Other risk factors can be divided into potentially modifiable and nonmodifiable. Nonmodifiable risk factors include dementia or cognitive impairment, age over 65, chronic renal or hepatic disease, multiple comorbidities, and a history of delirium, stroke, neurological disease, falls, or gait disorder. Potentially modifiable risk factors include surgery pain, intercurrent illness, acute neurological diseases, medications, immobilization even by catheters or restraints, sensory impairment of hearing or vision, metabolic derangements, environment, emotional distress, and sustained sleep deprivation. The categories of causes can be remembered by the mnemonic I WATCH DEATH. Table 8.2 explains the mnemonic.
Table 8.2
Causes of delirium
Categories | Examples |
|---|---|
Infectious | Encephalitis, meningitis, pneumonia, urinary tract infection |
Withdrawal | Alcohol, sedative-hypnotics |
Acute metabolic | Acidosis, alkalosis, electrolyte disturbances, hepatic or renal failure |
Trauma | Heat stroke, burns, surgery |
CNS pathology | Hemorrhage, seizures, stroke, tumors, vasculitis, hydrocephalus |
Hypoxia | Hypoxia from cardiac or pulmonary cause, anemia, carbon monoxide poisoning, hypotension |
Deficiencies | Vitamin B12, niacin, thiamine |
Endocrinopathies | Disorders of glucose, cortisol, thyroid, and parathyroids |
Acute vascular | Hypertensive encephalopathy, shock |
Toxins or drugs | Medications, toxins |
Heavy metals | Lead, manganese, mercury |
Dementia as a risk factor was discussed in the previous section. Two studies help accentuate its importance. Wahlund and Bjorlin in 1999 found that approximately 70 % of elderly patients admitted to a specialized delirium ward had either dementia or mild cognitive impairment [43]. In a study of total joint replacement patients, all demented patients postoperatively developed delirium, compared with 31.8 % in the non-demented patients [37].
Age is another risk factor for delirium. One study suggests this relationship is linear after age 65. In mechanically ventilated patients, the probability of developing delirium increased by 2 % for each year over 65 [44].
Hypoxia is a well-known risk factor for delirium. Not only poor oxygenation but also poor oxygen delivery, i.e., anemia, can contribute to delirium [45, 46]. Hypoxia can come from many causes, even obstructive sleep apnea [47].
Many medications have the capacity to cause delirium. This is especially true of those with psychoactive effects and those with anticholinergic affects. There appears to be a direct relationship between a drug’s anticholinergic properties and the development of delirium [48–50]. A study by Han et al. found that exposure to anticholinergic agents was an independent risk factor for the development of delirium and an increased symptom severity [51].
Dr. Mark Beers created the first Beers’ Criteria list in 1991with a consensus panel of experts. It has been updated several times, most recently in 2012. The list identifies medications or classes of medication that are potentially inappropriate that one should avoid in all older adults, in older adults with certain diseases and syndromes that the drugs listed can exacerbate, and medications to be used with caution in older adults [52]. The drugs which contribute to delirium are on this list. All practitioners caring for the elderly must be aware of this list and utilize it to the benefit of their patients. The most recent update of the criteria will be used as an educational tool and a quality measure.
Diagnosis and Screening
Delirium is unrecognized in many cases. Numbers in the literature range from 65 to 84 % of cases that go undiagnosed. Delirium can mimic many other mental illnesses. A cornerstone of diagnosis is looking for the underlying causes and correcting modifiable ones.
There are a host of objective diagnostic and screening tests for delirium in the literature (Table 8.3). In December 2008, the Canadian Agency for Drugs and Technologies in Health published a review of evidence-based guidelines on diagnostic tests for delirium. They reviewed the 2006 Canadian Coalition for Seniors’ Mental Health (CCSMH) published evidence-based guidelines for the assessment and treatment of delirium, the 2006 British Geriatrics Society and the Royal College of Physicians’ evidence-based delirium guidelines, and the 2007 delirium guidelines for general hospitals by Swiss and French physicians [55–58]. They concluded that early assessment for delirium is needed in hospitalized elderly patients. This early detection of risk factors may prevent delirium and its complication. Physicians and nurses must be trained to recognize delirium and educated in the use of validated screening and diagnostic tools. They suggested that the Confusion Assessment Method be used for screening and diagnosis and the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) criteria be used to confirm the diagnosis.
Table 8.3
Objective tests to diagnose delirium
Cognitive Test for Delirium (CTD) |
Confusion Assessment Method (CAM and Confusion Assessment Method for the Intensive Care Unit(CAM-ICU) |
Confusional State Evaluation (CSE) |
Delirium Assessment Scale (DAS) |
Delirium Detection Score (DDS) |
Delirium Index (DI) |
Delirium Rating Scale and Delirium Rating Scale-revised-98 (DRS) |
Delirium Severity Scale (DSS) |
Delirium Symptom Interview (DSI) |
Memorial Delirium Assessment Scale (MDAS) |
Short Portable Mental Status Questionnaire (SPMSQ) |
In 2010, the National Clinical Guideline Centre of Britain published its clinical guideline titled “Delirium: Diagnosis, Prevention and Management.” As to screening and diagnosis, they recommended screening for behavioral changes at presentation and daily during hospital admission. If indicators of delirium are identified, they recommend that a healthcare professional who is trained and competent in the diagnosis of delirium carry out a clinical assessment based on the DSM-IV criteria or Confusion Assessment Method short version (short CAM) to confirm the diagnosis. The CAM-ICU should be used when patients are intubated in the ICU or recovery room postoperatively [59].
Since the CAM and CAM-ICU have been recommended in these evidence-based reviews, we will discuss them here. The Confusion Assessment Method was unveiled in 1990 and the Confusion Assessment Method-ICU in 2001 [60, 61]. Both have a sensitivity of 94–100 %, a specificity of 89–95 %, and high inter-rater reliability [62].
The CAM has a long and a short version. The long version is comprehensive and screens for nine clinical features. The short version focuses on the four features that have the greatest discriminatory ability to detect delirium from other cognitive disorders (Fig. 8.1).
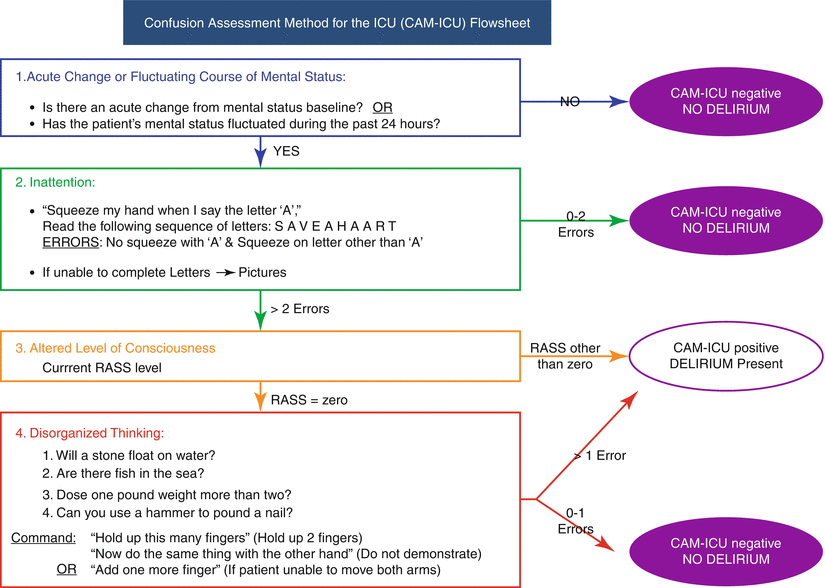

Fig. 8.1
Confusion Assessment Method for the ICU (CAM-ICU) flow sheet
The CAM-ICU addresses the same four areas as the short version CAM. It was developed by Ely et al. at Vanderbilt. It takes less than 2 minutes to complete and can be given to intubated patients. The process is shown in Fig. 8.1.
Prevention and Treatment
In the case of delirium, prevention is truly the best medicine. The National Clinical Guideline Centre of Britain addressed prevention in its clinical guideline titled “Delirium: Diagnosis, Prevention and Management.” [59] The following paragraphs are a summary of the findings of the review supplemented by additional sources as indicated.
Delirium can be difficult to recognize and treat. People at risk for delirium should be under the care of an interdisciplinary team that is “trained and competent in delirium prevention.” It would be best if the team were familiar to the patient at risk. Patients at risk should remain with the same caregivers and not change units unless necessary. A tailored multicomponent program should be administered which includes environmental change and non-pharmacological interventions. Rather than recommend a particular program, they chose to focus on the elements that should be addressed. They are detailed below. The early assessment of risk is key to this process.
Changes to ensure good sleep patterns include changes to the method of carrying out clinical care. Nursing and medical procedures should be avoided during sleep periods. This includes administration of medication as is possible. Noise should be reduced to a minimum during these times as well.
Attention must be paid to medications themselves. Utilization of a tool such as the Beers’ Criteria list mentioned earlier will help identify medications placing the patient at risk and suggest substitutes. Including a pharmacist on the interdisciplinary team can help.
Closely assessing for and correcting hypoxia are essential. The same is true for infection. Occult infection can present as delirium. Infection control procedures including reducing use of catheters are important.
Any sensory impairment that can be improved or resolved should be addressed. Removing impacted earwax and ensuring the availability and use of working hearing and visual aids are among the interventions that can reduce risk.
Lack of or impaired mobility is another risk factor that can be addressed. Early postoperative mobilization should be encouraged whenever possible. Assistive devices should be readily available. Even those who cannot walk should be encouraged to perform active range-of-motion exercises. Physical therapy or rehabilitation medicine can help as part of the interdisciplinary team.
Dehydration and constipation can be detrimental risk factors. Appropriate hydration can be maintained by oral, subcutaneous, or intravenous fluids depending on the status of the patient. Consult as necessary for patients with comorbidities such as heart or renal failure. A bowel regimen should be used with a stepwise approach for prophylaxis and treatment.
Nutrition must be maintained. Dentures should be properly fitting and available when needed.
Pain must be assessed by whatever means appropriate. Pain management should be undertaken if not already in place. Pain medication should be reviewed if already being administered.
Environmental and practice changes can address cognitive impairment and/or disorientation. Steps include appropriate lighting, clear signage, and easily visible clocks and calendars. As mentioned earlier, clear communication with eye contact should be the norm when dealing with these patients. Frequent reorientation and reassurance can help. Regular visits from family and friends and activities to stimulate cognition, such as reminiscing, have a positive impact as well.
When patients become agitated or a danger to themselves or others, verbal and nonverbal techniques should be used to de-escalate the situation. When these techniques fail, pharmacological interventions should be considered for short-term use. Haloperidol or olanzapine is recommended in the NICE Guideline. Again, per the geriatric medication mantra, “start low, go slow but go.” Cautiously titrate to symptoms. Particular caution must be used with antipsychotics for those with Parkinson’s-type diseases or Lewy body dementia if they are to be used at all.
One example of an algorithm for prevention and treatment was published by Maldonado in Critical Care Clinics 2008 [63]. Recommendations for the pharmacological treatment begin with assessing current medications and discontinuing inappropriate ones. If possible, only use benzodiazepines or barbiturates for CNS-depressant withdrawal, i.e., alcohol withdrawal, or when other recommended agents have failed and sedation is needed to prevent harm.
For the correction of central anticholinergic syndrome, consider acetylcholinesterase inhibitors. Serotonin antagonists (e.g., ondansetron) can be used to control serotonin elevations usually associated with hypoactive delirium.
Consider changing narcotics from morphine and meperidine to fentanyl or hydromorphone. Sleep can be promoted by melatonin or its agonists.
Choice and dose of agents differ depending on the type of delirium. For hyperactive delirium, low to moderate dose (<20 mg/24 h) haloperidol can be used after ascertaining there are no significant electrolyte abnormalities and other medications that prolong QTc or cardiac conditions. Haloperidol must be discontinued if QTc is prolonged to >25 % of baseline or >500 ms. Atypical antipsychotics should be considered in cases where haloperidol is contraindicated or not desirable. Maldonado suggests there is better evidence for risperidone and quetiapine and limited data for olanzapine, aripiprazole, and perospirone. Clozapine and ziprasidone should be avoided.
For hypoactive delirium, dopamine antagonists may have a place given the excess dopamine theory. Haloperidol may be used but in very low doses, 0.25 to 1 mg/24 h. If atypical antipsychotics are used, ones with low sedative properties should be used, like risperidone, unless sleep pattern is at issue. In cases of extreme psychomotor retardation or catatonia without agitation or psychosis, psychostimulant or conventional dopamine agonists can be of use.
Depression
Epidemiology
Depression in the elderly is under-recognized and undertreated. Almost one in five older adults who commit suicide have visited a physician within 24 h of their death, 41 % visited within 1 week of their suicide, and three-quarters of the elderly who commit suicide visited a physician within a month before their death [64, 65]. The incidence of major depression in the community of elderly is reported up to 1 in 20. This increases to 11.5 % in the hospitalized elderly and 13.5 % of those receiving home healthcare [66]. It is estimated that five million elderly have some symptoms of a depressive disorder [67]. There is a gender difference with the rate of depression higher for elderly women than men [68]. Depression also lowers life expectancy in this age group.
Periods of feeling blue, sad, or unhappy are normal. When these feelings persist and interfere significantly with the ability to function, they become abnormal. Major depressive disorder is diagnosed by DSM-IV-TR criteria when five (or more) of the symptoms in Table 8.4 have been present during the same 2-week period and represent a change from previous functioning [8]. At least one of the symptoms is (1) depressed mood or (2) loss of interest or pleasure. These symptoms must cause clinically significant distress or impairment in social, occupational, or other important areas of functioning. They cannot be a part of mixed episode and cannot be caused by the physiological effects of a substance or other general medical condition or better accounted for by bereavement.
Table 8.4




Symptoms of depression
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree