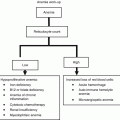
Component
Reason for assessment
Elements for assessment
Method of evaluation
Function
Functional status measures how independent a patient is and is a key factor for determining treatment success or failure
Activities of daily living (ADL)
Patient questions and then if relevant follow up
Instrumental activities of daily living (IADL)
Katz ADL scale [5]
Performance status (PS)
Lawton IADL scale
PS assessed using
Karnofsky Index
Eastern Cooperative Oncology Group (ECOG) [6]
Comorbidity
Serious medical conditions that are not directly related to the cancer should be evaluated and treated accordingly to maximise the benefits of therapy
Seriousness of comorbid conditions
Charlson Score [7]
Number of comorbid conditions
Cumulative Illness Rating Scale – Geriatric (CIRS-G) [8]
Socioeconomic factors
Socioeconomic issues may negatively affect compliance and thus treatment outcome
Living conditions
Social worker
Care support
General questions
Income and availability of financial counsel
Discussion with patient/caregiver
Access to transportation
Emotional and cognitive function
Cognitive impairment can jeopardize treatment safety and lead to worse prognosis. Special precautions should be taken to ensure participation and safety of these patients
Assessment of mental state (memory, orientation, comprehension, and logical thinking)
Orientation and counting tests
Depression is a condition common in both geriatric and oncology populations and can adversely affect treatment outcome
Evaluation of depressive state
Folstein Mini-Mental Status
Geriatric depression scale
Geriatric conditions
Geriatric problems should be identified and addressed to ensure maximum treatment benefits are achieved
Dementia, delirium, depression, falls, neglect
Geriatric depression scale
General questions
Nutrition
Malnutrition impacts on immune function and treatment tolerability
Weight and height
General questions
Weight loss
Nutritional risk: Mini-Nutritional Assessment
Polypharmacy
Potential drug–drug interactions could impact on patient well-being or treatment outcome; unnecessary drugs can be discontinued
Drug–drug interactions
General questions
Number of medications
Pharmacy assessment (collect all medicines)
Avoidance of inappropriate drugs
Comorbidity
Comorbidity has long been studied for its association with outcome in several malignancies. The most frequently used instruments are the Charlson comorbidity index (hereafter “Charlson”) and the CIRS-G [8, 18]. The properties of those instruments, as well as their advantages over ad hoc lists of diseases, has been discussed elsewhere [19]. Most of the studies included either all types of cancers confounded or solid tumors only. Some studies, however, have focused partially or entirely on hematologic malignancies. The presence of comorbidity has been analyzed as a prognostic factor for different end points, such as overall survival, in-hospital mortality, hospitalization, occurrence of toxicity, quality of life, and treatment allocation. One should note that some diseases have received more attention than others, without direct correlation with their relative prevalence.
Overall Survival
In a prospective cohort trial including patients with solid tumors (44.5 %) and hematological neoplasia (55.5 %), Wedding et al. identified comorbidity as an independent prognostic factor of survival (HR = 1.424; 95 % CI, 1.012–2.003) in addition to age (HR = 1.019; 95 % CI, 1.007–1.032), tumor type (HR = 1.832; 95 % CI, 1.314–2.554), and performance status (HR = 1.455; 95 % CI, 1.059–2.000) [20].
Acute Leukaemia
In a small retrospective series of acute leukemia patients (both AML and ALL), a New York team reported that patients with a comorbid condition involving a major organ had a worse median survival: 49 weeks for the group without comorbidity versus 20 weeks for the group with comorbidities. In patients above the age of 70, the figures were 32 weeks versus 4 weeks [21]. Etienne et al. retrospectively analyzed the treatment outcomes of 133 patients aged 70 years and older with AML treated with an intensive regimen. In a multivariate analysis, four adverse prognostic factors for CR and overall survival were identified: unfavorable karyotype, leukocytosis ≥30 g/L, CD34 expression on leukemic cells, and Charlson score >1 [22]. On the other hand, a retrospective analysis of treatment results in 92 patients aged 80 years and older diagnosed with AML found no significant correlation with 3-month survival of comorbidity, measured with either the Charlson index or the Sorror index [23, 24]. A recent study in 102 patient aged 60 and older found that in patients with favorable or intermediate cytogenetics, age <65, normal LDH, and an HCT-CI score of <3 were good prognostic factors for survival [25]. The authors created a simple prognostic index that still needs external validation.
Myelodysplastic Syndrome (MDS)
In a retrospective analysis of 419 patients with de novo MDS (median age 71 years), Sperr et al. compared two different comorbidity scores, the Hematopoietic Cell Transplant-Comorbidity Index (HCT-CI) and the Charlson index, for their association with overall and event-free survival (OS, EFS). The HCT-CI was associated with OS and with EFS, whereas Charlson was only associated with OS [26]. None of the indices was associated with transformation to AML. In multivariate analyses, comorbidity remained an independent prognostic factor of EFS and OS in patients with low or Int-1 MDS.
Hodgkin’s Disease
The SHIELD study enrolled 175 patients aged 60 and older (median 73) [27]. Patients were assessed with a modified ACE-27 comorbidity instrument. Patients having >3 G3 comorbidities or 1 grade 4/5 comorbidity were considered frail and did not receive the main study regimen: VEPEMB. They received treatment at the choice of the physician. Function was assessed with ADL, IADL, and ECOG PS. The VEPEMB treated patients had 74 % CR for early stage and 61 % CR for advanced stage. OS and PFS at 3 years were 62 % and 53 %. Among patients treated with curative intent (VEPEMB, ABVD, or CLVPP) none of the patients deemed frail achieved CR and all died with a median OS of 7 months.
Non-Hodgkin’s Lymphoma
Winkelmann et al. prospectively analyzed the prognostic value of CGA items in patients with malignant lymphoma. They identified the presence of severe comorbidity, assessed via the CIRS-G score, and IADL, as independent prognostic variables for survival [28]. In a cohort study by Lin et al., comorbidity by Charlson was not associated with the ability to receive complete treatment for DLCBL, but was associated with a decreased PFS and OS [29]. In a phase II study of DLCBL patients with moderate to high-risk cardiac disease treated with a CHOP-R regimen modified to replace doxorubicin with its non-pegylated liposomal form, patients with an age-adjusted Charlson score >7 had a shorter time to treatment failure [30].
CLL
A Mayo clinic team reviewed their CLL database for the influence of comorbidity on outcome [31]. The list of comorbidities was an ad hoc design, with six comorbidities defined as major. Data on ADL capacity were also available. The median age of the 373 patients was 67.6 years; 89 % of the patients had a comorbidity, and 46 % a major comorbidity; 8.9 % of patients had difficulty with their ADLs. On univariate analysis, the presence of a major comorbidity was associated with worse survival (P = .042). However, this effect was outweighed by Rai stage and age in the multivariate analysis. An interesting finding of this study was that 25 % of the patients would have been ineligible for a clinical trial based on typical eligibility criteria, but were treated nevertheless for their disease at similar times.
Intensive Care Unit (ICU) and Hospital Mortality
A study of patients admitted to the ICU for hematologic and solid tumors (mostly lymphomas and leukemias) identified Charlson score as an independent predictor of ICU and hospital mortality [32].
Febrile Neutropenia
Comorbidity was also associated with hospitalizations for neutropenia in older NHL patients. In the Iowa SEER/Medicare database, 21.7 % of patients aged 66 and older treated with chemotherapy had a hospitalization for febrile neutropenia; 41 % were hospitalized during the first cycle and 22 % during the second cycle. A positive Charlson score was an independent predictor with an associated adjusted hazard ratio of 1.50 (95 % CI, 1.03–2.17) [33]. A study in 1,355 community-treated patients of all ages treated with CHOP identified liver comorbity as a risk factor for hospitalization for febrile neutropenia, along with age >65, albumin <3.5, baseline absolute neutrophil count <1,500, planned dose intensity ≥80 % standard, and no early use of G-CSF [34]. A review of the 1992–2002 SEER data for the impact of prophylactic growth factors in 13,283 patients aged 65 and older (median 74.9) receiving chemotherapy for non-Hodgkin’s lymphoma recorded comorbidity according to the Klabunde index [35]. On multivariate analysis, comorbidity was associated with an increased risk of febrile neutropenia and documented infection, and a worse OS. The odds ratio (OR) was 1.23 (1.11–1.31) for a score of 1 and 1.15 (1.01–1.31) for a score of 2 or more for febrile neutropenia; 1.27 (1.16–1.39) for a score of 1 and 1.67 (1.50–1.86) for a score of 2 or more for infection. The OR of death was 1.28 (1.21–1.34) for a score of 1, and 1.75 (1.65–1.86) for a score of 2 or more. A retrospective study analyzed NHL and prostate patients treated at Moffitt for the impact of hyperglycemia on toxicity from chemotherapy [36]. The subgroup of 162 NHL patients were all treated with CHOP-rituximab (CHOP-R). Comorbidity, as measured with the CIRS-G, did not impact the occurrence of severe toxicity, whereas in prostate cancer patients the total CIRS-G score was associated with grade 3–4 nonhematologic toxicity. In NHL patients, grade 3–4 nonhematologic toxicity was associated with hyperglycemia, both at baseline and during treatment.
Quality of Life
An international study assessed the impact of comorbidity on the quality of life (QOL) of CLL patients: 1,482 patients answered a web questionnaire that included an evaluation of their Charlson score [37]. Overall, CLL patients had a similar QOL to that of the general population, but their emotional wellbeing scores were dramatically lower. Factors associated with lower overall QOL on multivariate analysis were older age, greater fatigue, severity of comorbid illnesses, and undergoing current treatment for CLL. Wedding et al. identified that elderly cancer patients with severe comorbidities (CIRS-G grade 3 or 4) had a worse quality of life, independently from functional status [38]. Forty-six percent of these patients had hematologic malignancies.
Treatment Allocation
An epidemiologic study by the Eindhoven cancer registry was conducted in Hodgkin’s and NHL patients aged 60 and older. It identified a prevalence of comorbidity by Charlson score of 58 % for NHL and 62 % for Hodgkin’s patients. This was associated with a decline in administration of chemotherapy and a 10–20 % decline in 5-year survival [39]..
Hematopoietic Cell Transplantation
Although transplant strategies are mostly offered to younger patients, transplantation is increasingly used in the fit elderly. Autologous transplant for multiple myeloma is a case in point. Fit patients in their lower seventies with high-risk disease are sometimes offered autologous or reduced intensity conditioning (RIC) allogeneic transplant. Wildes et al. compared patients aged 60 and over with younger patients for outcome of autologous stem cell transplant for relapsed NHL. They identified that comorbidity rated by Charlson score, rather than age, predicted transplant-related mortality and OS [40]. Labonté et al. compared the performance of the Charlson score, the HCT-CI [23], and the modified pretransplantation assessment of mortality (mPAM) in patients receiving autologous transplant for multiple myeloma [41]. All indexes performed similarly in being associated with serious organ toxicity and length of stay. Artz et al. compared the performance of Charlson and the Kaplan-Feinstein score (KFS) in patients receiving RIC for NHL (median age 52, range 17–70) [42]. The KFS was more sensitive and more strongly associated with transplant-related mortality. When combining the KFS and ECOG performance status (PS), a high-risk category could be identified. The patients with a KFS >2, and ECOG PS >1, or an alteration on both scores, had 4.1 times the risk of transplant-related mortality compared to the low risk group (50 % at 6 months vs 15 %). OS was also significantly reduced in older patients. Pollack et al. reviewed the impact of comorbidity measured by HCT-CI in NHL patients receiving RIC [43]. The median age was 53 years (range 32–74). Patients with 3+ comorbidities had more transplant-related mortality (26.3 % vs 4.5 % at 100 days, and 36.8 vs 13.6 % at 1 year). There was no association with disease-related mortality. Farina et al. similarly reviewed the association of HCT-CI with outcome in patients receiving RIC for lymphoma or myeloma [44]. HCT-CI was an independent determinant of OS (P < .001), PFS (P = .002), and non-relapse mortality (P = .03). Karnofsky PS was the other independent predictor for OS and non-relapse mortality. The effect was similar for lymphomas and myeloma.
Functional Status
ECOG performance status is a strong predictor of outcome in older AML patients. A large study from the Swedish Acute Leukemia Registry demonstrated several aspects of that relationship [45]. Older patients with a low PS score had more early deaths than those with good PS, no matter what their age. However, within each age and PS category, the patients who did receive intensive treatment had lower early death rates than the others (Table 13.2). Although they may represent selection bias, these results are consistent with those of a randomized EORTC study of immediate intensive chemotherapy versus observation followed by low-dose Ara-C [47]. Another study reviewed the safety of phase 1/2 clinical trials among AML patients older versus younger than 60 years [48]. Among 121 patients, there was no difference with age. However, ECOG PS was associated with both short-term and long-term survival. Patients with an ECOG PS of 0–1 had 97.5 % 30 days’ survival and 21 % 1 year survival, whereas patients with an ECOG PS of 2 or 3 had survivals of 79 and 9.5 % respectively. In NHL, ECOG PS has a well-established prognostic value as a component of the International Prognostic Index, with a score ≥2 being an adverse prognostic factor [49].
Therapy | |||||||||
|---|---|---|---|---|---|---|---|---|---|
All | Intensive | Palliative | |||||||
Age, years | ED | Total | %ED | ED | Total | %ED | ED | Total | %ED |
<50 | 15 | 342 | 4 | 14 | 336 | 4 | 1 | 6 | 7 |
50–54 | 14 | 160 | 9 | 12 | 155 | 8 | 2 | 5 | 40 |
55–59 | 25 | 181 | 14 | 17 | 165 | 10 | 8 | 16 | 50 |
60–64 | 27 | 242 | 11 | 20 | 223 | 9 | 7 | 19 | 37 |
65–69 | 43 | 308 | 14 | 20 | 246 | 8 | 23 | 61 | 38 |
70–74 | 83 | 419 | 20 | 35 | 281 | 12 | 47 | 137 | 34 |
75–79 | 98 | 448 | 22 | 30 | 202 | 15 | 67 | 244 | 27 |
80–84 | 125 | 411 | 30 | 25 | 96 | 26 | 100 | 312 | 32 |
85+ | 103 | 256 | 40 | 1 | 11 | 9 | 101 | 244 | 41 |
All groups | 533 | 2,767 | 19 | 174 | 1,715 | 10 | 356 | 1,044 | 34 |
WHO/ECOG PS 0-II | |||||||||
16–55 | 21 | 491 | 4 | 3 | 12 | 25 | |||
56–65 | 22 | 344 | 6 | 6 | 22 | 27 | |||
66–75 | 35 | 435 | 8 | 27 | 131 | 21 | |||
76–89 | 29 | 211 | 14 | 67 | 397 | 17 | |||
WHO/ECOG PS III–IV | |||||||||
16–55 | 10 | 38 | 26 | 2 | 4 | 50 | |||
56–65 | 12
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| ||||||||