The discovery of recurrent somatic genomic alterations in Langerhans cell histiocytosis (LCH) has led to a new understanding of LCH as a clonal neoplastic disorder. Most of the abnormalities described to date affect the RAS/RAF/MEK/extracellular-signal-regulated kinase (ERK) pathway: more than 50% of LCH cases carry activating mutations in BRAF , whereas another 10% to 28% carry activating mutations of MAP2K1 , which encodes MEK1. The pathogenetic importance of these mutations has been confirmed by reports of significant clinical responses to RAF inhibitors.
Key points
- •
Recurrent somatic genomic abnormalities occur in Langerhans cell histiocytosis (LCH), indicating that it is a neoplastic disease.
- •
Most mutations activate signaling enzymes that result in extracellular-signal-regulated kinase (ERK) activation.
- •
More than 50% of cases carry BRAF mutations and 10% to 28% carry MAP2K1 mutations, but all cases show activation of ERK.
- •
Significant clinical responses to RAF family inhibitors have been reported in patients whose LCH cells carry BRAF mutations, indicating that these mutations are authentic drivers of disease in LCH.
Introduction
Long considered an enigmatic disease, LCH defies simple categorization. Its 4 clinically distinct syndromes (Hand-Schüller-Christian disease, Letterer-Siwe disease, eosinophilic granuloma, and Hashimoto-Pritzker disease) were unified by observations in the mid-twentieth century of a characteristically abnormal histiocyte with distinctive morphology, subcellular structures, and staining patterns that appears in all forms of the disease. Although this categorization has helped clarify thinking about LCH, it has not explained its pathogenesis or its impressively protean clinical manifestations.
Further progress in characterizing LCH has been impeded by a paucity of samples, a consequence both of the low prevalence of LCH and the small size of most tissue samples obtained for clinical purposes. However, technical advances in the genomic analysis of clinical material have been applied to LCH and have revolutionized the understanding of the fundamental nature of the disease. One of the most enabling innovations is the ability to perform robust multiplexed genetic testing on small amounts of archived clinical material, that is, formalin-fixed, paraffin-embedded samples acquired for diagnostic purposes. This ability has opened the archives of pathology departments around the world to genomic analyses, and the application of these technologies has revealed some of the first evidence for recurrent and pathogenetically relevant mutations in LCH. To date, these have been somatic mutations rather than germline alterations affecting risk. The result has been a clearer understanding of LCH as a neoplastic disease and the identification of therapeutically important molecular targets.
Introduction
Long considered an enigmatic disease, LCH defies simple categorization. Its 4 clinically distinct syndromes (Hand-Schüller-Christian disease, Letterer-Siwe disease, eosinophilic granuloma, and Hashimoto-Pritzker disease) were unified by observations in the mid-twentieth century of a characteristically abnormal histiocyte with distinctive morphology, subcellular structures, and staining patterns that appears in all forms of the disease. Although this categorization has helped clarify thinking about LCH, it has not explained its pathogenesis or its impressively protean clinical manifestations.
Further progress in characterizing LCH has been impeded by a paucity of samples, a consequence both of the low prevalence of LCH and the small size of most tissue samples obtained for clinical purposes. However, technical advances in the genomic analysis of clinical material have been applied to LCH and have revolutionized the understanding of the fundamental nature of the disease. One of the most enabling innovations is the ability to perform robust multiplexed genetic testing on small amounts of archived clinical material, that is, formalin-fixed, paraffin-embedded samples acquired for diagnostic purposes. This ability has opened the archives of pathology departments around the world to genomic analyses, and the application of these technologies has revealed some of the first evidence for recurrent and pathogenetically relevant mutations in LCH. To date, these have been somatic mutations rather than germline alterations affecting risk. The result has been a clearer understanding of LCH as a neoplastic disease and the identification of therapeutically important molecular targets.
Mitogen-activated protein kinase pathway activation
BRAF
One of the first technologies capable of testing archived human samples for multiple alleles simultaneously was the Sequenom mass spectrometric genotyping platform. A modification specific for oncology applications known as OncoMap, which tests 983 specific mutations in 115 cancer-related genes, was applied to 61 LCH clinical samples. Overall, very few mutations were detected, a common finding in all subsequent studies (see later discussion) attesting to the stability of the LCH genome. However, a mutation in BRAF encoding the substitution of glutamate for valine at amino acid 600 (BRAF V600E) was observed in 57% of the samples. This mutation, which produces a constitutively active BRAF kinase, is the most commonly observed BRAF variant in cancer and is found in a variety of different cancer types in which it often plays a driver role in pathogenesis. The effect of mutationally activated BRAF is to stimulate signaling through the RAS/RAF/MEK/ERK pathway leading to constitutive transcription of genes involved in a variety of cellular responses including proliferation ( Fig. 1 ).
The presence in LCH of recurrent activating mutations in BRAF has been confirmed in several studies using a variety of different detection techniques ( Table 1 ). Among them are immunohistochemical studies with a V600E-specific antibody, which confirms that the variant is present specifically in LCH histiocytes, a fact that could previously be inferred only indirectly by molecular means. BRAF mutations occur in all clinical settings, including pediatric, adult, single system, and multisystem disease, and their overall prevalence in reported studies is 45% to 65%. Surprisingly, BRAF mutations appear at a nearly similar frequency in pulmonary LCH, a disease of adult smokers that has generally been thought to be polyclonal. However, about one-third of pulmonary LCH cases are clonal. It is also possible that cases that are polyclonal in the aggregate actually comprise several clones of BRAF mutant disease that arose independently.
| Report | Prevalence a (%) |
|---|---|
| Badalian-Very et al, 2010 | 57 (35/61) 42 (5/12) pulmonary only 61 (30/49) extrapulmonary |
| Haroche et al, 2012 | 38 (11/29) |
| Sahm et al, 2012 | 38 (34/89) b , c |
| Satoh et al, 2012 | 56 (9/16) c |
| Wei et al, 2013 | 56 (28/50) 100 (1/1) pulmonary only 55 (27/49) extrapulmonary |
| Roden et al, 2014 | 33 (26/79) 28 (7/25) pulmonary only 35 (19/54) extrapulmonary |
| Berres et al, 2014 | 64 (64/100) c |
| Chilosi et al, 2014 | 46 (18/38) 63 (12/19) pulmonary only 32 (6/19) extrapulmonary |
| Méhes et al, 2014 | 53 (8/15) |
| Varga et al, 2014 | 54 (6/11) adult cutaneous |
| Bubolz et al, 2014 | 48 (23/48) 25 (1/4) pulmonary only 50 (22/44) extrapulmonary |
a Prevalence is indicated with actual numbers shown in parentheses (number of cases with mutated BRAF /total number of cases).
b Detected by immunohistochemistry using VE-1 antibody.
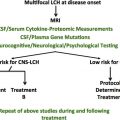
In the original OncoMap analysis, the median age of patients whose histiocytes contain BRAF V600E was less than the median age of patients whose histiocytes did not, and younger age was associated with the presence of the mutation in an unadjusted exact logistic model but not in the adjusted model. The presence of mutated BRAF did not correlate with any other clinical features, although the clinical annotation of that sample set was limited. In contrast, in the largest sample set analyzed to date, clinical annotation was much more complete and the presence of BRAF V600E correlated with disease relapse. That study also identified BRAF V600E in bone marrow–derived hematopoietic precursors from patients with high-risk LCH. In patients with low-risk disease, the mutation was detected only in lesional cells. This finding suggests that the appearance of a driver mutation earlier in ontogeny might lead to a more widely disseminated and aggressive form of LCH.
Like other BRAF-driven diseases, LCH is also associated with activating mutations of BRAF other than V600E. For example, another acidic amino acid substitution for V600, BRAF V600D (a substitution of aspartate for valine), is an activating mutation that occurs rarely in melanoma and has been reported in a single case of LCH. A more commonly observed BRAF variant in melanoma, BRAF V600K (a substitution of lysine for valine), has not yet been reported in LCH. In addition, an insertional mutation substituting an additional 4 amino acids, DLAT (aspartate-leucine-alanine-threonine), for valine at amino acid position 600 has been found in a single LCH case. Although this variant has not been tested directly for constitutive kinase activity, structural inferences suggest that its effects on constitutive activation of BRAF kinase should be similar to that of V600E.
ARAF
Although activating BRAF mutations are present in 45% to 65% of LCH cases, the histiocytes in all LCH cases examined to date show high levels of phosphorylated ERK, implying ERK pathway activation (see Fig. 1 ). This finding has led several groups to perform even broader analyses such as whole-exome sequencing to find mechanisms for ERK activation other than activating BRAF mutations. A surprising outcome of one whole-exome analysis was the discovery of an activating mutation of ARAF . ARAF is another mitogen-activated protein (MAP) kinase kinase kinase in the same protein family as BRAF ( Fig. 2 ). The ARAF abnormality discovered in this study was actually a compound mutation comprising a single base substitution encoding leucine in place of phenylalanine at position 351 (F351L) and a 6-nucleotide deletion leading to loss of amino acids 347 and 348 (Q347_A348del). Biochemical analysis of this variant demonstrated that it is a constitutively active kinase capable of transforming mouse embryo fibroblasts, suggesting that it is likely to be a driver mutation in this clinical case. This ARAF variant was sensitive to inhibition by vemurafenib in vitro.
A distinct ARAF mutation, T70M (methionine substituted for threonine at position 70), has also been described in a single mixed case of LCH and Erdheim-Chester disease (ECD). Although this variant, which appears in the COSMIC (Catalog of Somatic Mutations in Cancer) database, has not been tested for RAF kinase activity, it is unlikely to be activated because it appeared in a case that also carried a BRAF V600E mutation. To date, no mutations in CRAF (also known as RAF1) have been described in LCH.
MAP2K1
Ongoing sequence analysis of LCH samples has uncovered additional somatic mutations that activate the ERK pathway. Most prominent are activating mutations in MAP2K1 , which encodes the MAP kinase kinase MEK1 (see Fig. 2 ). These mutations are found only in cases carrying wild-type BRAF alleles, supporting the notion that they are acting in the same signaling pathway as BRAF. The true prevalence of MAP2K1 mutations is uncertain at present because their frequency differs substantially among the samples tested in various reports: 28% in a 40-sample cohort, 19% in a different 36-sample cohort (of a 41-sample collection that included some mixed LCH/ECD and LCH/juvenile xanthogranuloma cases), and 10% in a 30 sample cohort. It is possible that this disparity arises from differences in the clinical characteristics of the patients who comprised these cohorts, but the presence of MAP2K1 mutations does not correlate with age, sex, sites of disease, or stage in these studies.
MAP2K1 mutations in LCH cluster vary specifically in 2 domains: an N-terminal negative regulatory domain and the N-terminal portion of the core kinase domain ( Table 2 ). Some MAP2K1 mutations found in solid tumors, leukemias, or lymphomas overlap these regions, but many more occur throughout the protein. Among the mutations in LCH are several that result in the same alterations of the MAP2K1 protein but involve different nucleotide substitutions (see Table 2 ). For example, the substitution of serine for cysteine at position 121 (C121S) is created both by a substitution of G for C at nucleotide position 362, an alteration also observed in melanoma, and by a substitution of A for T at position 361, creating a differently mutated codon that nonetheless still encodes serine at amino acid 121. Similarly, the deletion of amino acids 58 through 62 arises through an in-frame deletion of nucleotides 303 through 308 or 304 through 309. Finally, the frequently observed deletion of glutamate at position 102 (E102) and isoleucine at position 103 (I103) is produced through in-frame deletions starting at nucleotides 302, 303, or 304. This finding almost certainly reflects substantial selective pressure for these particular variant proteins, which can arise through a variety of alterations at the DNA level, which may be context- or patient specific.