Fig. 14.1
Triple immunostain cocktail of a prostate biopsy. In the definitely benign prostate acini (top), a layer of basal cells is stained brown, representing reactivity for cytokeratin 34βE12, a cocktail of cytokeratins 1, 5, 10, and 14, and for nuclear p53 reactivity. There is no pink staining for AMACR (p504S, racemase) in the top acini. The acini toward the center react differently with only a few, focal basal cells present and weak, pink racemase reactivity, while showing little, if any, cytologic atypia. The cluster of acini toward the center illustrates the occasional problem faced but prostate pathologists, because the findings are suspicious for, but not diagnostic of, adenocarcinoma
CpG Island Hypermethylation Profile: GSTP1 Methylation
GSTP1 (glutathione S-transferase pi 1) methylation is perhaps the best studied epigenetic marker in prostatic adenocarcinoma [2]. The gene encodes GSTP1 protein, which functions as a prominent tumor suppressing detoxifying agent, preventing genomic damage by carcinogens. Methylation of GSTP1 was first reported as a specific biomarker for prostatic cancer in 1994 [3]. Since that time, more than 30 independent studies have reported both high sensitivity and high specificity in detecting prostatic adenocarcinoma. The utility of gene hypermethylation analysis is generally reserved for cases in which there is a high clinical suspicion for prostate cancer, but initial prostate biopsy is negative. Rather than attempt a re-biopsy, the histologically noncancerous tissue can be evaluated for the presence of hypermethylated genes associated with the presence of prostate cancer. These genetic alterations can be seen in histologically nonmalignant tissue adjacent to unsampled cancerous tissue, which is known as a “field effect.” A meta-analysis performed by Van Neste et al. 2012, showed that testing for GSTP1 methylation both retrospectively and for biopsy verification yielded a combined sensitivity and specificity of 82 % and 95 %, respectively [4].
In one pivotal study, the MATLOC study, hypermethylation of APC and RASSF1 was used in combination with GSTP1 [5]. Results were interpreted such that at least 1 gene in at least 1 tissue core produced a positive assay result, and the methylation ratio of all the three genes was determined relative to beta-actin reference gene. The study demonstrated that methylation analysis on initially negative biopsies serves as a significant independent predictor of the presence of unsampled prostatic adenocarcinoma. Other variables, including subject age or race, or the presence of atypia or high grade PIN (HGPIN), were not significantly correlated with the presence of unsampled malignancy. The overall negative predictive value of the combined methylation analysis was 90 %, which showed good agreement with the NPV documented in other studies. Currently, this methylation test for the three genes is marketed as ConfirmMDx (Table 14.1).
Table 14.1
Comparison of available tests for prostate cancer
Test | Specimen | Uses | Company |
|---|---|---|---|
PSA | Blood | Since the late 1980s, the standard screening test for prostate cancer; is nonspecific, as 2/3 of men with elevated PSA do not have cancer. | Various |
Prostarix | Urine | Screening: tests for sarcosine and other metabolites | Metabolon |
Prostate Health Index | Blood | Screening: tests for variant forms of PSA | Beckman Coulter |
Mi-Prostate Score (MiPS) | Blood and urine | Screening: serum PSA, urine TMPRSS2:EFG, and urine PCA3 | Univ. of Michigan MLabs |
Progensa PCA3 | Urine | Screening: Tests ratio of PCA3 expression to PSA | Metamark |
Confirm MDx | Tissue | After a negative biopsy, when suspicion persists, tests for epigenetic field effect on methylation of the three genes if cancer were nearby | MDxHealth |
Prolaris | Tissue | After a biopsy, tests activity of a panel of genes | Myriad Genetics |
ProstaVysion | Tissue | After a biopsy with low-intermediate grade cancer, tests for PTEN loss and ERG, to predict progression | Bostwick Laboratories |
ProMark | Tissue | PTEN loss + ERG, as above | Metamark |
Genomic Prostate Score (GPS) | Tissue | After a biopsy, tests activity of a panel of genes | Genomic Health |
Prognostic Tissue Markers
HOXD3 Methylation
Homeobox D3 (HOXD3), is a gene whose silencing by methylation was shown to correlate with tumor grade [6]. By methylation-specific PCR, it was associated with biochemical recurrence in univariate analysis (P-value = 0.043) and showed evidence for interaction with pathological stage as a predictor variable in Cox regression analysis (P-value = 0.028) [6]. It is not being used as a commercial test currently.
Serum and Urinary Markers
Serum PSA, a surrogate marker for prostate cancer, is a sensitive test for prostate cancer but lacks specificity: two-thirds of men undergoing prostate biopsy on the basis of elevated serum PSA will have benign histology. In the last 5–10 years, many new tests have emerged (Table 14.1).
The PCA3 test is a urine-based nucleic acid amplification assay, detecting PCA3, a noncoding mRNA which is selectively overexpressed in prostate cancer cells as a ratio to the expression of PSA mRNA which serves as a marker of prostate cells. A test score ≥ 35 is considered to warrant a repeat biopsy. The PCA3 test has relatively lower sensitivity of 49 % in the largest study (58–74 % in other studies), but its great strength is its specificity, of 78 % in that study [7]. This test is mostly used to supplement serum PSA findings. Its use was approved in Europe in November 2006, and it is now commercially available from many laboratories. In the USA the test became available commercially as uPM3 and has been available since May 2006. It was cleared by the FDA in 2011, and is currently marketed as Progensa PCA3 (Hologic Gen-Probe, Inc).
To perform the test, the urologist collects the first 20–30 ml of voided urine after careful digital rectal examination. 2 ml urine is transferred into a transport tube with lysis buffer, followed by overnight urine specimen shipment to testing center with frozen gel packs. The mean PCA3 score ranges from 30 to 50 and shows no correlation with prostate volume. PCA3 was shown by Whitman et al. [8] to predict both prostate cancer stage and volume. It was significantly higher in men with extraprostatic extension (49 vs. 19); showed a positive correlation with cancer volume (r = 0.38); and by multivariate analysis was an independent predictor of EPE and cancer volume. PCA3 + biopsy Gleason Score + PSA gave a receiver operator characteristic of 0.90 (for EPE). Thus, PCA3 can be used after a negative set of biopsies to determine which men need immediate repeat biopsy and which are candidates for active surveillance.
The MLabs at University of Michigan have developed the Mi-Prostate Score test which combines PCA3 testing with urinary TMPRSS2-ERG fusion (discussed below) and serum PSA for maximal specificity in predicting prostate cancer and whether the cancer is high grade [9, 10].
PHI
Another new, related test is the Prostate Health index or PHI [11] from Beckman Coulter. The PHI is a new formula that combines three kallikreins (total PSA, free PSA, and p2PSA—the benign form of PSA or BPSA) into a single score that can be used to aid in clinical decision-making. Rhodes et al. [12] note that although the PHI test offers statistically significant results when applied to large populations, the clinical utility of the PHI test for individual patient management is not yet determined. The authors’ carefully worded conclusion states that “The PHI measurement, … may be useful to decrease unnecessary biopsies with improved specificity at various sensitivities for [prostate cancer] detection in men 50 years or older with PSA 2.0–10.0 ng/ml and negative DRE findings”.
Pro-PSA
PSA and its derivatives are kallikreins. [−2] proPSA (p2PSA) is a subtype of free PSA (the benign form of PSA), also called pro-PSA, and is estimated to be 2.5 times more specific in detecting prostate cancer in patients than a PSA screening [13]. It was approved by the US Food and Drug Administration (FDA) in June 2012 and has been available since late 2012.
Sarcosine
Sarcosine is markedly elevated in metastatic prostate cancer and moderately elevated in the urine from primary prostate cancer and its biosynthetic enzyme is elevated in prostate cancer tissues [14]. Also, sarcosine was found to introduce invasion and intravasation of prostate cancer cells in an in vivo prostate cancer model. Prostarix, is a non-invasive urine test introduced in 2013 [15] that involves metabolic assessment using a quantitative liquid chromatography-mass spectroscopy method to accurately measure the urinary concentrations of four amino acids. This test is considered an alternative method of deciding whether to perform a set of prostate biopsies in men with mildly elevated serum PSA and negative digital rectal examination—in other words, men at equivocal risk.
Prognostic Tissue Markers
PTEN and ERG
In needle biopsy specimens, the prostate cancers for which a prognostic test is indicated are those with a highest Gleason score of 3 + 3 = 6 or 3 + 4 = 7. The test may tip the therapeutic response toward watchful waiting or cryotherapy rather than definitive therapy. However, cancer with a score of 4 + 3 = 7 or above is presumed aggressive and a test is not indicated. A number of American laboratories perform testing for the two most adverse prognostic markers in prostate pathology: PTEN loss and ERG expression. ERG is an androgen-responsive gene. Many studies have reproducibly shown that about 50 % of prostate cancer acquires reactivity for ERG, reflecting TMPRSS2-ERG rearrangement. About 16 % of high-grade prostatic intraepithelial neoplasia (HGPIN), the noninvasive precursor to invasive cancer [16] Carver] also is ERG-positive, and benign prostate never is. Tomlins et al. [17] showed that the most common rearrangement is TMPRSS2-ERG (21q22.2) followed by TMPRSS2-ETV1 (7p21.2), while TMPRSS2 (21q22)-ETV4 (17q21) (17q21) is rare (2 % of cases). Klezovich et al. [18] established a causal role of ERG overexpression in carcinogenesis. Overexpression of ERG in prostate cell lines increased cell invasion. Targeted expression in vivo in luminal prostate epithelial cells of transgenic mice resulted in development of focal precancerous prostatic intraepithelial neoplasia (PIN) [18] Klezovich]. Multifocal prostate cancer can show heterogeneity of ERG expression [19 Park].
ERG expression alone has only a marginal [20 Yoshimoto et al.] or no [21 Hoogland] effect on outcome as measured by biochemical recurrence-free survival. PTEN (Phosphatase and tensin homolog deleted on chromosome 10) is a key tumor suppressor gene in prostate cancer. Loss of expression of PTEN occurs in about one-third of prostate cancer [22 McCall].
PTEN deletions were present in 20.2 % (458/2,266) of prostate cancer (8.1 % heterozygous and 12.1 % homozygous [23 Krohn et al.]. PTEN deletions were linked to advanced stage, high Gleason grade, presence of lymph node metastasis, hormone-refractory disease, and presence of ERG gene fusion (all P < 0.0001). PTEN deletions were associated with biochemical recurrence in both univariate (P < 0.0001) and multivariate (P = 0.0158) analyses [24]. Given these findings, many private practice urologists, are electing to have PTEN loss study performed reflexively for their newly diagnosed cancers with Gleason scores of 3 + 3 = 6 or 3 + 4 = 7 in order to stratify the otherwise heterogeneous outcomes of these grade categories.
Lotan et al. [25] have investigated PTEN protein loss by immunohistochemistry to compare its assessment with that of FISH. PTEN immunostaining (IHC) detected up to 86 % of cases with PTEN genomic loss by comparison with single nucleotide polymorphisms. IHC was 87 % sensitive for hemizygous PTEN deletion, and 86 % sensitive for homozygous deletion. It was also found by IHC at times in the absence of apparent genomic loss. That is, in 37 % of cases in which SNP did not demonstrate PTEN loss and 45 % of cases in which FISH did not demonstrate PTEN loss, it was found by immunostaining. Others have reported similar findings in comparing IHC with FISH [26, 27]. Taken together, these data suggested that PTEN inactivation can occur by means other than genomic deletion, including insertions and epigenetic changes; and PTEN detection by IHC may hold some advantage over FISH. The main drawback, however, is that IHC cannot discriminate hemizygous and homozygous loss.
While ERG expression alone is not important alone, it becomes a significant prognostic factor only when combined with PTEN loss. Kaplan-Meier analysis shows that the status of these two markers can stratify men with prostate cancer into four significantly different groups [20]. Carver et al. [16] showed that in combination, ERG and PTEN define three groups of patients by prognosis: a good prognosis: ERG-/PTEN+ (29 % of cases); intermediate prognosis: ERG+/PTEN+ or ERG-/PTEN-(43 %); and poor prognosis: ERG+/PTEN- (28 %).
A special type of prostate cancer that is receiving abundant attention today is intraductal carcinoma (IDC) and the molecular biology peculiar to IDC has been reviewed [28]. IDC is an inherently high-grade type of prostate cancer, wherein duct spaces are distended to at least twice the diameter of a normal duct and contain a lumen-spanning proliferation of cells with marked nuclear enlargement, sometimes with luminal necrosis. The rates of ERG fusion and PTEN loss in IDC are at least commensurate with those of invasive carcinoma, and exceed the corresponding rates for HGPIN. Fine et al. [29] found no difference in ERG translocation/deletion or copy number increase in tumors from men with or without IDC. Immunoreactivity for ERG protein, reflecting a TMPRSS2-ERG gene fusion, has been reported in 35–75 % [30] of IDC but rarely or never in atypical cribriform lesions, which in the case of a topographic study using proximity to invasive cancer to discriminate IDC, [31] provides a molecular justification for topography being discriminatory. Schneider and Osunkoya found that the presence or absence of ERG reactivity in IDC always matched that of the acinar carcinoma; but the 35 % rate of expression of the invasive carcinoma associated with IDC was less than that not associated with IDC, suggesting that when IDC is present, the accompanying invasive component tends to have a unique phenotype [32] Schneider]. ERG immunostaining may be diagnostically useful in select cases. Cytoplasmic PTEN loss has been suggested as a marker to distinguish IDC from HGPIN, being observed in 84 % of IDC and 100 % of lesions intermediate between IDC and HGPIN, but never in HGPIN [28]. Nuclear reactivity for PTEN may be retained in IDC. IDC’s rate of PTEN loss exceeds the 35–45 % rates reported for acinar carcinoma [25, 33–36] but is similar to the significantly higher PTEN loss in Gleason grades 4–5 cancer [34], supporting IDC’s aggressiveness.
Is ERG immunostaining useful for diagnosis as well as prognosis? Andrews and Humphrey [2] showed that ERG reactivity is contributory only in uncommon cases of minimal adenocarcinoma when AMACR staining is negative or weak. Even then, in these cases ERG is informative in only a minority (29 %) of cases. Warrick and Humphrey [37] have also studied ERG expression in foamy gland carcinoma, which represents 17 % of cases. Sensitivity of AMACR for foamy gland carcinoma was 92 %, and sensitivity of ERG was 42 %. No AMACR-negative case was ERG positive. Low AMACR expression was detected in 24 of 72 cases (33 %), and of these cases, ERG was positive in 5 (21 %). This suggests that ERG is not useful as a first-round immunostain for foamy gland carcinoma and may only be useful as a supplementary marker in rare cases if AMACR is weak.
PSMA
PSMA (FOLH1), has been studied as an immunohistochemical marker and has been found to predict progression in a statistically independent manner when controlling for tumor grade, stage, and clinical factors. In one study [38], PSMA expression and metastases to pelvic lymph nodes were significantly associated with time to PSA recurrence (HR, 1.4; 95 % confidence interval, 1.1–2.8, P = 0.017; and hazard ratio, 5; 95 % confidence interval, 2.6–9.7, P < 0.001, respectively). However, this marker is used mainly in a research capacity.
Other Prognostic Markers
Survival pathways such as nuclear factor kappa B, MYC (c-MYC), phosphorylated AKT, its downstream effector FKHR (FOXO1) have all been studied in prostate cancer. Interestingly, all these markers correlated with the diameter of perineural invasion, in which a symbiotic relationship with nerves causes growth of both the cancer cells and the nerves [39 Olar et al]. These findings validate the importance of reporting perineural invasion when seen in prostatic biopsy cores.
Cell adhesion proteins mediate interactions of epithelial cells to each other and to the stroma, and thus cancer progression and metastasis. IDC shares in common with cribriform/papillary, large acinar (LA) cancer a dense concentration of neoplastic cells with absent intervening stroma and a uniquely adverse outcome. Invasive cribriform carcinoma, compared to other Gleason grade four patterns, was present in 61 % of men who experienced PSA failure but only 16 % of controls matched for follow-up duration and other clinicopathologic variables [40]. The quality of the stromal component of prostate cancer has been recognized as a prognostic factor. Indeed, loss of expression of integrins β1 in the epithelium and β3 in the stroma were noted in intraductal and cribriform cancer xenografts and untreated human tumors, compared with their non-cribriform counterparts [41]. This provides a rationale to expect that cribriform lesions—with or without surrounding basal cells—may be expected to show altered expression of other cell adhesion proteins.
Oncotype Dx
Genomic Health, Inc. produces Oncotype Dx [42], a proprietary test of prostate cancer tissue for a series of genomic alterations pertaining to four pathways that drive prostate cancer. This furnishes a Genomic Prostate Score (GPS) that is said to predict outcome after surgery, regardless of what postoperative therapy is chosen. The original development studies looked at a subset of 185 prostate cancer patients who experienced biochemical recurrence after surgery.
Prolaris
Myriad Genetics markets a prognostic test for low (3 + 3 = 6) to intermediate (3 + 4 = 7) grade cancer called Prolaris, that uses a proprietary panel of gene expression to predict prognosis.
Markers Whose Expression Correlates with Morphology
The most widely studied of these cell adhesion markers is CD44. CD44 is a cell-stroma and cell-cell adhesion and signaling protein, of which the standard isoform is decreased in prostate cancer, while variant isoforms get overexpressed owing to aberrant mRNA splicing, including exons coding for CD44v7-10, and this has functional significance for growth and invasion [43]. Recent papers have demonstrated that the presence and amount of cribriform (or large acinar) pattern as a component of prostate cancer confers a distinct disadvantage in outcome, making both PSA failure and metastasis after prostatectomy more likely [30, 44].
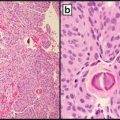
Iczkowski et al [30] performed immunostaining for CD44 variant 7/8 (clone VFF-17, SeroTec, Raleigh, NC) at a 1:50 dilution with citrate retrieval, on 15 prostatectomy tissues. Other biomarkers with established relevance to high-grade prostate cancer including RB1, hTERT, were stained for in the same tissues. The only protein showing differential marking of Gleason 4 patterns was CD44v7. Diminished CD44v7 reactivity in cribriform acini as compared with fused small acini from the same case was noted in 13 of 15 cases (87 %). Figure 14.2 shows loss of cytoplasmic CD44v7/8 in the cribriform structure, particularly central cells, compared with fused small acini from the same slide (inset). Interestingly, alterations of CD44 standard and variant isoforms showed a role in growth and invasion of tumor [43], suggesting that the degree of altered cell interactions, with cell dyshesion, as manifested by CD44 or other adhesion markers, correlates with tumor growth morphology.
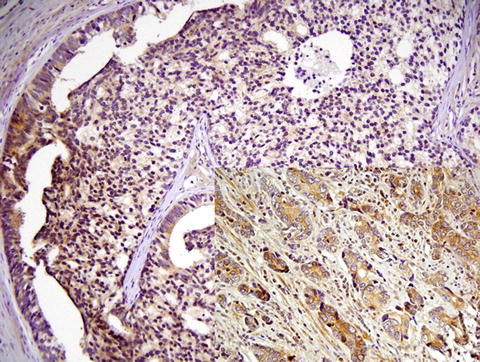

Fig. 14.2
Cribriform cancer also portends a worse outcome than fused small acini, yet both morphologies currently get assigned a Gleason grade of 4. There is less cytoplasmic reactivity for CD44 splice variant CD44v7/8 in the cribriform structure, particularly central cells, compared with fused small acini from the same slide. This suggests that alterations in splice isoforms of the cell adhesion molecule CD44 play a role in altered morphology
Intraductal carcinoma (IDC) also has its own morphologic correlates. Allelic instability in IDC exceeds that of HGPIN or Gleason grade 3 cancer. IDC had at least double the rates of loss of heterozygosity for 12 microsatellite markers [31] and TP53 and RB1 [45] compared to invasive acinar carcinoma or HGPIN. Ki-67 also appeared to correlate with IDC patterns [45]. The Ki-67 proliferative index of IDC was 11.1 ± 3.7 %; this was commensurate with that of invasive carcinoma and significantly higher than the 1.5 ± 0.7 % index for HGPIN [45], suggesting that Ki-67 is the most useful differential diagnostic marker to date.
Markers for Drug Targeting
PSMA
PSMA (GCPII, FOLH1) is an enzyme encoded by folate hydrolase (prostate-specific membrane antigen) 1 (FOLH1). It is expressed in many tissues, including the prostate, kidney, the small intestine, and the central and peripheral nervous system. Unlike PSA, whose expression per cell decreases in cancer compared with benign prostate and which decreases in the highest grades of prostate cancer, the expression of PSMA increases with about a tenfold-higher expression in prostate cancer than benign prostate. PSMA therefore has found use as a diagnostic immunohistochemical stain and PSMA is the target of an approved imaging agent for prostate cancer, capromabpentide, PROSTASCINT (Cytogen Corporation, Princeton, NJ). Various antibodies and ligands are being developed to target PSMA [46, 47].
ERBB2 (HER2)
ERBB2 (HER2), best known for its predictive value in breast cancer, is to a lesser degree prognostic in prostate cancer. 17–20 % of reactive cases are reactive in conventional prostatic adenocarcinoma. Interestingly, however, 65 % percent of prostate cancers with intraductal carcinoma (IDC) are immunoreactive for ERBB2 in the IDC, suggesting that such cases may benefit from targeted Herceptin antibodies [38]. However, because ERBB2 (HER2) reactivity increases with higher Gleason score per se, it is still not known whether the IDC has any greater reactivity than accompanying invasive high-grade acinar components. Another tumor that is in the differential diagnosis for IDC is adenoid cystic/basal cell carcinoma, and that tumor was consistently strongly reactive for ERBB2 [48] so the distinction with IDC must be made morphologically.
Testis and Epididymis
Most of the differential diagnosis in challenging testicular neoplasms relies on immunohistochemistry, although there are some uses for molecular genetics. In the past decade, Cheng et al. [49] have developed stem cell marker OCT3/4 as the most useful marker for distinguishing seminoma and embryonal carcinoma from all other tumors. A few other markers such as CD117 [50] and SALL4 [51] have also been added to traditional markers (Table 14.2).
Table 14.2
Immunohistochemical profiles of testicular germ cell tumorsa
Tumor | OCT3/4 | CD117 (c-kit) | CKb | hCGa | Vimentin | PLAP | SALL4c |
|---|---|---|---|---|---|---|---|
Seminoma, IGCNU | + | +d | ± | ± | + | + | + |
Spermatocytic seminoma | − | +d | −/+ | − | − | ± | |
Embryonal carcinoma | + | − | + | − | + | + | |
Yolk sac tumor | − | − | + | + | + | + | |
Teratoma | − | − | + | + | ± | ± | |
Choriocarcinoma | − | − | + | + | − | ± | − |
In some instances, the germ cell origin of testicular tumors is in doubt and must be distinguished from sex cord-stromal tumors. The analysis of chromosome 12p by FISH can be used to establish germ cell origin of the tumor [53]. Amplification of 12p, particularly in the form of isochromosome duplication, is a common feature shared by the vast majority of testicular germ cell neoplasms, of all histologic types [53].
Typically, metastatic germ cell tumors will maintain their characteristic histologic features, or show features of other germ cell neoplasms not apparent in the primary tumor. In rare cases, somatic-type histologic features will predominate in metastasis, making it difficult to distinguish between a primary somatic tumor and a metastatic germ cell tumor. Identification of chromosome 12p amplification in this context can be a useful diagnostic tool.
Though standard cytogenetics can be used to evaluate 12p amplification, FISH has been shown to be a more sensitive modality, detecting amplification in 88–100 % of tumors in several studies [54–56].
Kernek et al. [53] evaluated 11 cases of somatic type tumors in patients with a history of testicular or mediastinal germ cell neoplasms. Using interphase FISH, they identified 12p amplification in 6 of the 11 cases, helping to confirm testicular origin. Of note, this study performed FISH studies on formalin-fixed, paraffin-embedded (FFPE) tissue, which was sectioned at 4 μm thickness. This approach was used to prevent significant nuclear overlap while ensuring that visible nuclei would contain all chormosomes. Using standard thickness PPFE tissue may result in false negative results due incomplete chromosomal material being present on the slide.
As with other germ cell neoplasms, overexpression of chromosome 12p is a defining feature of postpubertal teratomas. In contrast to their ovarian counterparts, testicular teratomas are by convention considered malignant neoplasms. However, a small subset of cases which lack this characteristic molecular feature may show a benign clinical course. Zhang et al. [57] published a series of 25 cases of dermoid cysts and nondermoid teratomas, in which chromosome 12p amplification was not detected. Of the 17 patients for whom follow-up information was available, all were alive at postoperative intervals of 5–168 months. Histologic evaluation of the orchiectomy specimens showed no evidence of cytologic atypia. Areas of adjacent testicular parenchyma showed no evidence of intratubular germ cell neoplasia (IGCN), tubular atrophy/sclerosis, microlithiasis, or impaired spermatogenesis. These changes are characteristically seen in the majority of malignant teratomas of the testis. The authors argue that a new category of “benign-type” testicular teratoma should be considered. This diagnosis would be predicated on absence of 12p overexpression, as well as the absence of these parenchymal changes commonly seen in malignant cases.
Testicular germ cell tumors are one of the few human tumors in which serum markers are part of the AJCC-approved TNM staging system. In adults, yolk sac tumor and choriocarcinoma almost always occur as part of mixed germ cell tumors, whereas in children, they occur in pure form. The specific serum markers for yolk sac tumor and choriocarcinoma respectively are alpha-fetoprotein (AFP) and human chorionic gonadotropin (hCG). Lactate dehydrogenase (LDH) is the least specific according to tumor type, and may be elevated in all germ cell tumors, particularly in mixed germ cell tumors.
SX: marker studies not performed
S1: LDH 1.5 times “normal” (upper limit of normal for assay) and hCG <5,000 mIμ/mL, and AFP <1,000 μg/mL
S2: LDH 1.5–10 times “normal” or hCG 5,000–50,000 mIμ/mL, or AFP 1,000–10,000 μg/mL
S3: LDH 10 times “normal” or hCG >50,000 mIμ/mL, or AFP >10,000 μg/mL
The most common tumor of the epididymis is adenomatoid tumor. Immunohistochemistry can help establish the differential diagnosis [58] and rule out papillary cystadenoma/carcinoma of the epididymis, or invasion by a primary testicular tumor. Cells are mesothelial and strongly react with cytokeratins AE1/3, CK7, CAM5.2, calretinin, WT1, and vimentin. Lack of reactivity for vascular markers rules out hemangioma and angiosarcoma. Low miB-1 proliferation index and lack of cytokeratin 20 reactivity rule out gastrointestinal and other primary origins. Notably, tumor cells can be reactive with germ cell marker D2-40, a diagnostic pitfall.
Urinary Bladder and Upper Urinary Tract
Background
The urinary bladder is the most frequent site of cancer in the luminal urinary tract, and the sixth most common cancer in the USA, with an annual incidence of approximately 75,000. According to the SEER factsheet, there are an estimated 571,518 people living in the USA with a diagnosis of bladder cancer, with 15,000 deaths/year [59]. The classical and most common presenting symptom of bladder cancer is painless, intermittent hematuria. Other clinical features include urinary urgency and frequency, incontinence, and dysuria or other obstructive symptoms. Signs suggestive of an invasive malignancy include renal failure, a palpable suprapubic mass, as well as the classical systemic symptoms of weight loss and anorexia.
Tobacco use has been established as the single most important risk factor for the development of bladder cancer. Burch et al. reported that the incidence of transitional cell carcinoma of the bladder is approximately four times higher in smokers than in nonsmokers [60]. Other risk factors include cyclophosphamide use, history of pelvic radiation, and chronic irritation associated with infection such as Schistosomiasis, or the use of long-term indwelling catheters.
Primary urothelial neoplasms of the bladder are broadly categorized as flat or papillary lesions, and further subclassified as invasive or noninvasive. As with epithelial neoplasms in other organs, urothelial carcinomas of the bladder may be low- or high-grade, which may harbor variable molecular and chromosomal aberrancies, and require different diagnostic algorithms. Treatment largely depends on stage and grade; superficial tumors are usually treated with transurethral resection and vesicular chemotherapy/pro-inflammatory agents, while deeply invasive tumors are treated with radical cystectomy and systemic chemotherapy.
Cytoscopy and urine cytology have long been the standard method for monitoring recurrence. Cystoscopy, while highly sensitive, is also an invasive and costly test. Cytology is minimally invasive, but demonstrates a sensitivity of only 44 %, and may be as low as 27 % for low-grade tumors [61].
Molecular Tests
FISH
Tumors are associated with various genetic and epigenetic mutations occurring at both the chromosomal and molecular level, and result in oncogene activation and tumor suppressor gene inactivation. Types of tumor-associated chromosomal abnormalities include amplification, deletion, aneuploidy and translocation. FISH, or Fluorescence In-Situ Hybridization, is technique that utilizes fluorescently labeled DNA probes to detect these chromosomal abnormalities. There are two types of FISH probes: chromosome enumeration probes (CEPs) and locus-specific indicator (LSI) probes. CEPs are used to detect aneuploidy, whereas LSI probes are typically used to detect chromosomal amplification, duplication, or deletion.
FISH is used to detect recurrent abnormalities characteristic of a variety of neoplasms, including cancers of the lung, pancreatobiliary tract, esophagus, and bladder. There are at least two systems which currently offer FISH for bladder neoplasms, performed on urine and bladder washings:
Urovysion and Cellay
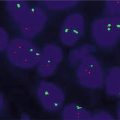
UroVysion
UroVysion (Abbott Molecular Inc, Des Plaines, IL), was the first commercially available FISH assay available for bladder tumor detection, performed on voided urine samples. The assay was introduced by Sokolova et al. in 2000, and utilizes a 4-target, multicolor probe set, containing CEP probes for of chromosomes 3, 7, and 17, and one LSI probe for loss of 9p21 locus [62]. These targets are labeled with red, green, aqua, and gold fluorophores, respectively. Nonneoplastic urothelial cells are expected to show two copies for each of the four probes. Occasionally, signal overlap or incomplete hybridization may cause normal cells to show only one copy of one or more of the four. Urothelial carcinoma cells, on the other hand, will show one of several types of chromosomal abnormality, which include polysomy, tetrasomy/near-tetrasomy, trisomy, and 9p21 loss.
Although voided urine is the FDA-approved specimen type, UroVysion can be performed on any type of urine, including urine obtained by catheterization, bladder washings, and stomal specimens. Combined Urovysion FISH and conventional cytology can be complementary with the poor sensitivity but high specificity of cytology being compensated for by the high sensitivity of FISH; FISH is thus most useful reflex test after an equivocal cytologic diagnosis [63].
Cellay
The Cellay system is an alternative FISH assay which has the advantage of same-day diagnosis, as opposed to the overnight incubation required by UroVysion. Instead of genomic DNA fragments, Cellay utilizes small oligodeoxynucleotide (ODN) FISH probes, targeted at defined centromeric repeat sequences. These ODNs have superior hybridization kinetics and lower manufacturing costs compared to traditional genomic probes [64]. This results in faster hybridization (5 minutes), compared to high complexity probes (8–16 h). A drawback according to some opinions is that the small size of ODN probes limits the number of labels that can be used, and accordingly limits the sensitivity of the assay.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree