, John W. Davis2, Jeri Kim1, Karen E. Hoffman3, William E. Osai1 and Deborah A. Kuban3
(1)
Department of Genitourinary Medical Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA
(2)
Department of Urology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA
(3)
Department of Radiation Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA
Chapter Overview
Genitourinary (GU) cancer encompasses several cancers, occurring in both the young and the old, and affecting predominantly men. Survivors of GU cancers constitute more than 50% of male cancer survivors, and primary care clinicians and specialists will likely see an increasing number of GU cancer survivors in their practice. Long-term cancer survivors have ongoing needs, and evidence-based guidelines can assist busy clinicians in understanding and confidently managing these needs. This chapter summarizes the needs of long-term GU cancer survivors, using evidence-based cancer-specific algorithms. The algorithms were developed for the purpose of addressing the many needs of long-term survivors, on the basis of the Institute of Medicine’s survivorship model of care.
Introduction
Genitourinary (GU) cancer is a diverse group of diseases that includes prostate, testicular, bladder, kidney, and penile cancer. The population affected is heterogeneous in terms of age at diagnosis, risk factors, treatment, and prognosis, although the most common characteristic is male sex. According to the Institute of Medicine (Hewitt et al. 2005), more than 50% of male cancer survivors are survivors of GU cancer. The high rate of GU cancer survivorship is a testament to the success of treatment modalities, but long-term monitoring of these patients presents a challenge for two main reasons: (1) a broad knowledge base is needed because of the heterogeneity of GU cancers, and (2) recommendations for surveillance beyond 3 years are inconsistent or lacking and fail to address quality-of-life issues and management of late effects specific to each type of cancer (Hewitt et al. 2005).
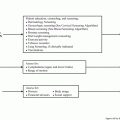
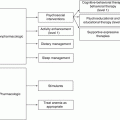
The GU survivorship clinical practice algorithms included with this chapter provide an evidence-based standard of care for long-term survivors of GU cancer who have completed treatment at least 2–5 years previously and show no evidence of recurrent disease. The algorithms were developed by translating existing evidence into recommendations under four concurrent components of the survivorship visit. A more detailed description of the models of care can be found in Chap. 2. A discussion of each disease-specific algorithm will be the focus of the remainder of this chapter.
Kidney Cancer (Renal Cell Carcinoma)
Renal cell carcinoma (RCC) accounts for up to 90% of kidney cancer. Several histologic types of RCC exist, but 85% are classified as clear cell carcinoma (DeVita et al. 2008). Other histologic types include papillary, chromophobe, and collecting duct; sarcomatoid differentiation is associated with poor outcome (National Comprehensive Cancer Network 2011). RCC incidence has steadily increased since 1975, and the increase is attributed mostly to early-stage disease found incidentally (National Cancer Institute 2011). Environmental risk factors include tobacco use, obesity, and hypertension. Hereditary conditions such as Von Hippel-Lindau disease account for only a small percentage of RCC diagnoses (American Cancer Society 2012).
Surgical resection of the primary tumor remains the standard of care for localized tumors, and this treatment results in cure for most low-grade, early-stage tumors. Radical nephrectomy, used to treat locally advanced tumors, involves removal of the kidney, adrenal gland, perirenal fat, and Gerota fascia. Nephron-sparing partial nephrectomy is performed in select cases. Lymphadenectomy may also be performed (National Cancer Institute 2011). Newer, less invasive surgical approaches such as radiofrequency ablation and cryotherapy are offered to some patients, especially those for whom a traditional surgical approach is too risky.
Prognosis with RCC is inversely correlated with both stage and grade at diagnosis. Five-year overall survival rates with RCC range from 5% to 94%, depending on stage, histologic findings, and source of the malignancy (Chin et al. 2006). The Fuhrman nuclear grading system is used to grade clear cell tumor morphology. Grades range from 1 to 4, and grade is inversely correlated with prognosis independent of stage (Chin et al. 2006). The University of California Los Angeles Integrated Staging System is a validated system that places patients into low-, intermediate-, and high-risk categories on the basis of tumor stage and grade.
Surveillance and Eligibility for Care at the MD Anderson Survivorship Clinic
Most patients who are eligible for care in our survivorship clinic have been treated definitively with partial or radical nephrectomy. However, a few patients have been treated with systemic therapy such as interferon, interleukin-2, or, more recently, molecular targeted therapies. Patients with hereditary RCC (Von Hippel-Lindau disease) are not eligible because of the high recurrence rate associated with this disease. Patients whose primary treatment modality was an ablative therapy are also not eligible because of the lack of reported 5-year survival and recurrence rates associated with this treatment. Although cases of late metastases from RCC are documented, 93% of recurrences occur within 5 years of nephrectomy. The most common metastatic sites are lungs, bones, liver, and renal fossa (Shuch et al. 2012). Lung lesions and local recurrence can be detected by imaging of the chest and abdomen. Liver metastases can be diagnosed by findings on computed tomographic (CT) imaging and elevated transaminases or bilirubin levels. Bone metastases most often occur within the first 3 years after completion of treatment and generally manifest as pain and elevated alkaline phosphatase levels. Therefore, routine surveillance of bones is not recommended for long-term survivors of RCC (Chin et al. 2006; Shuch et al. 2012).
The role of CT imaging during the long-term phase of survivorship is not as clear as it is in the first 2–3 years after therapy, when the risk of recurrence is much higher. The risks of CT imaging must be balanced by its contribution to the management of recurrences and complications because the cumulative dose of radiation from frequent CTs can be significant, especially in young patients. Additionally, the nephrotoxic effect of contrast dye in a patient with a solitary kidney must be considered, as well as the unnecessary expense and anxiety created from false positive findings. The type and frequency of diagnostic studies recommended in the algorithm are therefore based on risk of recurrence and metastatic patterns of RCC, as well as potential late effects of treatment.
Annual visits are recommended between years 5 and 15 after treatment is completed, and during each visit a thorough history and physical examination is essential. Although occult metastases can occur in the absence of symptoms, many patients with recurrence have constitutional or organ-specific symptoms. A CT scan is omitted in patients with low-grade, low-risk T1 tumors. After year 10, imaging is performed only as clinically indicated.
Late Effects: Monitoring and Management
Renal insufficiency is the main adverse effect of treatment because most patients undergo nephrectomy as the primary therapy. Even when creatinine levels are normal, a patient with a solitary kidney is at risk for renal compromise if exposed to certain conditions. The patient must be educated regarding his or her role in the prevention and management of this lifelong treatment effect. Nephrotoxic medications are particularly dangerous, and patients must be instructed to minimize or avoid use of nonsteroidal anti-inflammatory drugs. Depending on the glomerular filtration rate, medications for the treatment of intercurrent illnesses and chronic conditions may require dose reduction.
Blood pressure control is imperative. Hypertension can be prevented through lifestyle modification but may require pharmacotherapy. Weight management protects renal function, not only directly through reduction in body mass, but also by reducing the risk of diseases such as diabetes and hypertension that are associated with kidney disease. Adequate hydration is also important, especially if the patient must undergo diagnostic studies that use nephrotoxic contrast media. Renal function is easily monitored by annual laboratory testing of blood urea nitrogen and creatinine. Many patients with kidney cancer have a significant comorbidity that already predisposes them to renal dysfunction, and after nephrectomy, they often meet the criteria for stage II–III chronic kidney disease (Chapman et al. 2010). These patients need more frequent creatinine monitoring and may require referral to a nephrologist. Systemic therapy such as interferon, interleukin-2, and, more recently, molecular targeted therapies are generally reserved for patients with advanced RCC. In the rare case that a patient is disease-free 5 years after chemotherapy, he or she will likely require surveillance that exceeds the recommendations of the current kidney cancer survivorship algorithm.
Urothelial Cancer: Bladder/Ureter/Renal Pelvis
Urothelial cell carcinoma of the bladder (transitional cell carcinoma) is associated with a wide variety of clinical behaviors and responses to treatment. In simple terms, the disease is categorized into a low-grade/low-stage “superficial” form and a high-grade/progressive-stage form. The superficial form is more of a nuisance cancer in that cystoscopic resection is generally successful, but recurrent tumors are common, requiring surveillance with periodic cystoscopy.
For patients with invasive disease who undergo extirpation of the bladder, recurrence and progression are significant problems, occurring in approximately 50% of patients by 2 years after surgery. Recurrence beyond 2 years is increasingly uncommon. Patients undergoing cystectomy also undergo a pelvic lymph node dissection and a urinary diversion. Urinary diversions can be categorized in three common categories: conduit (incontinent), continent catheterizable reservoir, and continent neobladder.
Surveillance and Eligibility for Care at the MD Anderson Survivorship Clinic
Patients with superficial bladder cancer are not eligible for the survivorship care outlined in bladder/ureter/renal pelvis cancer survivorship algorithm (presented at the end of the chapter) owing to the nature of recurrences and need for frequent endoscopic surveillance. Most patients entering the survivorship clinic have been treated definitively with cystectomy and urinary diversion. A small percentage of patients have received systemic chemotherapy in the adjuvant or neoadjuvant setting. Annual surveillance recommendations take into account the probability and locations of disease recurrence, with the goal of optimizing quality of life and managing possible deterioration of renal function or metabolic abnormalities from the urinary diversion.
Metastatic recurrence is very rare at 5 or more years after completion of treatment, and follow-up should emphasize functional status (Jaske et al. 2006). New primary urothelial tumors may occur in the upper tract or retained urethra; however, vigorous imaging-based screening is of unclear benefit as opposed to standard evaluation for bleeding or other clinical presentations (Sanderson and Roupret 2007). In a review by Studer et al. (2006), urethral recurrence after orthotopic substitution occurred in 25 (5%) of 482 cases, with a median time to recurrence of 14 months (range 3–158 months). Upper tract recurrence was diagnosed in 15 (3%) of 482 cases at a median of 31 months (range 12–72 months). Intravenous pyelogram is recommended every other year between years 5 and 10 after completion of treatment for monitoring and management of functional issues and new upper tract lesions, with the addition of a CT urogram if clinically indicated. The diagnostic yield of urine cytology is controversial, but urine cytology remains the standard of care in most guidelines (National Comprehensive Cancer Network 2011).
It is also noteworthy that the most common risk factor for bladder cancer is smoking, and therefore survivors of this disease may experience additional smoking-related health concerns and cessation needs.
Late Effects: Monitoring and Management
Although cancer recurrence is not anticipated after 5 years, declining functional status, stoma issues, and bowel complications may occur. The simplest form of urinary diversion is the ileal conduit, in which the ureters are attached to a 15–20-cm isolated segment of the distal ileum and the distal end is exteriorized as an incontinent diversion to an external appliance. Madersbacher et al. (2003) reported that the overall conduit-related complication rate was 66%. The 5-year complication rate was 45%, but this increased to 50% at 10 years, 54% at 15 years, and 94% at more than 15 years after surgery. The main causes of complications were upper tract changes (hydronephrosis) and urolithiasis. For stoma-related problems (e.g., hernia), the median time to development of the complication was 54 months (range 4–274 months); most occurred within the first 5 years. However, stomal stenosis occurred in 6% of patients in this series. Bowel-related complications also occurred mostly within the first 5 years.
Shimko et al. (2011) analyzed another large single-institution cohort of 1,057 patients who underwent either ileal or colonic conduit urinary diversion. The cumulative rate of complications was 60.8% (643 patients), and 1,453 complications were attributable to the conduit. Incidence rates for complications were as follows: bowel, 20.3%; renal, 20.2%; infectious, 16.5%; stomal, 15.4%; urolithiasis, 15.3%; metabolic, 12.8%; and hydronephrosis, 11.5%. In terms of follow-up length, of 276 patients surviving for more than 5 years after surgery without complications, 116 (42%) eventually experienced a complication. Renal replacement therapy was necessary in 26 patients (2.5%) at a median of 8.4 years (range 0.9–23.5 years), and another 22 patients (2.1%) experienced loss of a functional renal unit at a median of 2.4 years (range 0.2–23.5 years).
Patients selected for orthotopic neobladder urinary diversion are generally younger and more fit than those who undergo ileal conduit urinary diversion. However, stage of disease and predicted survival are not necessarily the driving selection factors; younger patients are less likely to suffer adverse effects from complications of the procedure. Therefore, non–disease-related mortality rates may be decreased in the short term by selecting younger patients. Both populations (i.e., those who undergo neobladder urinary diversion and those who undergo ileal conduit urinary diversion) also undergo a significant primary surgery to remove the bladder and stage the lymph nodes, and in both populations, the bowel is reconstructed and subject to leakage, fistula, and obstruction at any time in the future. The key difference is that approximately 40 additional centimeters of ileum are harvested for a neobladder urinary diversion.
Long-term and short-term metabolic changes occur after a urinary diversion procedure. The extent of these changes depends on the type of procedure and length of bowel used for the diversion. In Studer et al. (2006), a common postoperative management decision for patients who underwent an ileal neobladder urinary diversion was administration of 2–6 g of oral sodium bicarbonate daily to prevent acidosis. Rehospitalization occurred in 30 (6.2%) of 482 patients. Of the patients surviving beyond 10 years after surgery, none had to continue the bicarbonate therapy. Patients surviving 15–20 years after surgery had bone densities matching those of age-matched controls. Vitamin B12 levels were subnormal in 37 (12%) of 314 patients, and 15 (5%) received vitamin B12 replacement therapy. Comparing two series from the same surgeon (Madersbacher et al. 2003; Studer et al. 2006), one could conclude that the upper tract preservation and metabolic acid disturbance rates were not significantly different between the two urinary diversion techniques. Choice of the neobladder construction technique or ileal conduit diversion is a matter of surgeon preference and training, in addition to patient preference. Regardless of the surgical technique used, clinicians should monitor patients for bone demineralization, electrolyte imbalance, and bowel symptoms if clinically indicated.
In general, urinary continence is satisfactory after neobladder urinary diversion, but never as good as prior to surgery. In the Studer series (Studer et al. 2006), overall daytime continence was 92%, achieving a plateau at 12 months and remaining stable for 7 years. Nighttime continence rates were lower—79%—and many used an alarm clock to ensure at least one nighttime void. Hypercontinence is possible, especially in woman undergoing a neobladder urinary diversion. In the Studer series, 7% of patients used intermittent self-catheterization or an indwelling catheter.
Erectile function rates are difficult to capture in patients with urothelial cancer owing to multiple comorbidities and age. In the Studer series, 99 (22.4%) of 442 evaluable men reported at least one successful erection without medical assistance and 68 (15.4%) reported at least one with medical assistance. In clinical practice, many patients with aggressive disease are not selected for a nerve-sparing procedure, and therefore support for erectile dysfunction needs should be anticipated (see Chap. 25 on sexuality).
Some patients receive chemotherapy, generally platinum-based. These patients may experience long-term effects such as prolonged bone marrow suppression, peripheral neuropathy, or renal insufficiency, and symptoms should be managed accordingly. The algorithm recommends annual blood urea nitrogen and creatinine testing; an annual complete blood count is also reasonable for monitoring purposes.
Urothelial Cancer: Upper Tract
Patients surviving upper tract urothelial cancer show a heterogeneous range of outcomes. Their follow-up issues reported more than 5 years after completion of treatment are not significantly different from those of patients with RCC who undergo nephrectomy, and most issues involve management of the remaining renal unit. Before the 5-year mark, patients undergo frequent cystoscopic evaluations of the bladder because bladder recurrence may be as high as 50%. For patients with high-grade tumors, management is similar but is more likely to involve definitive resection if the patient has a normal contralateral kidney. Many of these procedures are performed using laparoscopic techniques, and the time-honored tradition is to resect the entire ureter and a cuff of bladder where the ureter enters. Muntener et al. (2007) reviewed a contemporary series of upper tract disease managed with laparoscopic techniques and found that the overall results were similar to those in patients undergoing open surgery.
Appropriate surveillance and care for survivors of transitional cell carcinoma of the upper tract is covered by the bladder/ureter/renal pelvis cancer survivorship algorithm. Surveillance tests for transitional cell carcinoma of the upper tract from years 5 through 20 after completion of treatment parallel surveillance tests for bladder cancer, with the addition of annual monitoring of electrolyte levels. The late effects of treatment are similar to those noted in the kidney cancer survivorship algorithm.
Prostate Cancer
Prostate cancer is the second-most common cancer diagnosed in men worldwide and is the most common cancer diagnosed in men in developed countries (Jemal et al. 2011). In 2013, an estimated 238,590 men will be diagnosed with prostate cancer in the United States and 39,720 men will die from the disease (American Cancer Society 2012). Since the introduction of prostate specific antigen (PSA) screening in the early 1990s, most men with prostate cancer are diagnosed with disease that has not spread beyond the prostate and immediately surrounding tissue. Men with clinically localized disease are categorized into low-, intermediate-, and high-risk groups on the basis of tumor (T) stage, Gleason score, and PSA level. Treatment decisions are guided by extent of disease, other medical conditions, and patient preference. Common treatments for prostate cancer include active surveillance, surgical resection, brachytherapy, external beam radiation therapy, and androgen deprivation therapy. Some men receive treatment that combines two or more of these treatment modalities.
Survival after treatment is typically long-term. For all stages, the 5-year survival rate is 99%, the 10-year survival rate is 95%, and the 15-year survival rate is 82% (American Cancer Society 2012). Because of the long-term survival, men often live with the medical and psychosocial effects of treatment for multiple decades. The likelihood and character of medical late effects of treatment vary with the treatment received; late effects may include urinary incontinence, rectal bleeding, and impotence.
Surveillance and Eligibility for Care at the MD Anderson Survivorship Clinic
The prostate cancer survivorship algorithm (presented at the end of the chapter) addresses care of men who have completed treatment for prostate cancer at least 2 years previously and show no evidence of disease. To be eligible for care at the survivorship clinic, men who underwent prostatectomy must have a PSA level of less than 0.1 ng/ml and men who received radiation therapy must have a PSA level of less than 1.0 ng/ml that is not rising.
Prostate cancer survivors are evaluated annually for disease recurrence, late effects of treatment, and psychosocial distress. Although most men treated for localized prostate cancer do not develop recurrent disease, recurrence can arise many years after treatment. Men with high-risk features at initial diagnosis are at increased risk for disease recurrence, but all men should be followed for possible recurrence because early detection and treatment of recurrence may improve outcomes.
At the annual evaluation, clinicians should perform a general physical examination and digital rectal examination and determine PSA levels to evaluate for disease recurrence. Testosterone levels are determined for select men if clinically indicated, including men whose testosterone level did not return to normal after androgen deprivation therapy. A rise in PSA levels or an abnormal digital rectal examination may trigger further diagnostic workup. If the patient is found to have recurrent disease, he should be referred back to his primary treating oncologist.
Late Effects: Monitoring and Management
Because the survival duration after prostate cancer is typically long, men can live with the late effects of treatment for multiple decades. Medical late effects of treatment include sexual, urinary, and bowel dysfunction. The likelihood and character of late effects depend on the treatment. Men who underwent prostatectomy are more likely to have urinary incontinence, whereas men who received radiation therapy are more likely to have rectal symptoms (Sanda et al. 2008). In addition to undergoing assessment of potential sexual, urinary, and bowel late effects of treatment, men who received androgen deprivation therapy should be evaluated for possible bone and endocrine effects of therapy.
Erectile Dysfunction
Sexual dysfunction, both erectile and orgasmic, is common after both prostatectomy and radiation therapy (Penson et al. 2003). Please see Chap. 25 on sexuality for more information about the treatment of erectile and orgasmic dysfunction. Therapy for erectile dysfunction includes phosphodiesterase-5 inhibitors, penile self-injection programs with vasoactive drugs, vacuum erection devices, and penile prosthesis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree