ADENOMATOUS POLYPOSIS
Familial Adenomatous Polyposis and Attenuated Familial Adenomatous Polyposis
Of all of the colonic polyposis conditions, FAP is both the most common and the best characterized. FAP is caused by germ-line mutations in the adenomatous polyposis coli (APC) gene and is estimated to occur in about 1 in 10,000 individuals. With the classic presentation of FAP, hundreds to thousands of adenomatous polyps occur by the age of 20 to 40 years.1 The attenuated or less severe colonic phenotype associated with AFAP may mimic sporadic colon polyps and cancer, or other known syndromes, such as MAP. This creates diagnostic difficulties when evaluating an individual with moderate adenomatous polyposis. Other conditions linked to germ-line APC mutations include Gardner syndrome (with association of colonic polyposis and osteomas, epidermoid cysts, fibromas, and/or desmoid tumors) and Turcot syndrome (with association of colonic polyposis and medulloblastomas).2 However, it is now believed that the features associated with Gardner syndrome and Turcot syndrome are the result of variable expressivity of APC mutations as opposed to being distinct clinical entities.
Colon Phenotype
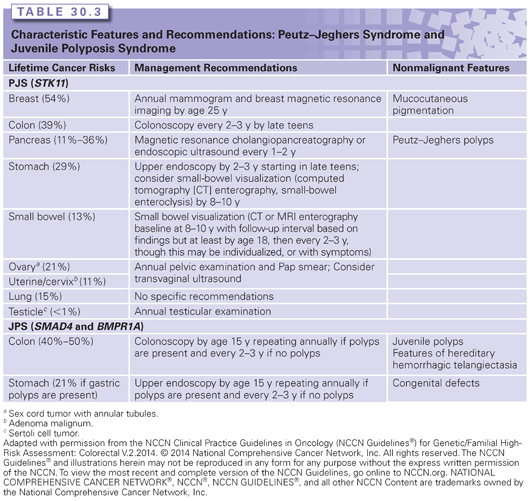
Although adenomatous polyps associated with FAP have a similar malignancy rate as those that develop in the general population, the sheer number of polyps present in FAP results in nearly a 100% lifetime risk of CRC in untreated individuals. In FAP, colorectal polyps begin to develop on average around the age of 16 years.1 The mean age at CRC onset is 39 years, with 7% developing CRC by 21 years and 95% before the age of 50 years.3
In AFAP, the lifetime risk of CRC is approximately 70% with an average age at onset in the 50s.4 The colonic phenotype of AFAP is quite variable, even within the same family. Colonoscopies in 120 mutation-positive individuals within the same family revealed that 37% had less than 10 adenomatous colon polyps (average age, 36 years; range, 16 to 67 years), 28% had 10 to 50 polyps (average age, 39 years; range, 21 to 76 years), and 35% had greater than 50 polyps (average age, 48 years; range, 27 to 49 years).4 In addition, the total number of polyps per individual ranged from zero to 470.4
Extracolonic Features
The most common extracolonic finding in individuals with FAP and AFAP is upper gastrointestinal tract polyps. Although the colonic phenotype in AFAP is less severe than in FAP, the upper gastrointestinal phenotype is comparable. Adenomatous polyps of the duodenum (20% to 100%) and the periampullary region (at least 50%) are common.5,6 The relative risk of duodenal or periampullary carcinoma in FAP is estimated to be 100 to 330 times greater than the general population, although the absolute risk is only around 5%.5 The majority of FAP- and AFAP-associated small-bowel carcinomas arise in the duodenum.
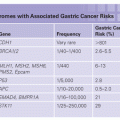
Fundic gland polyps are found in most cases of FAP/AFAP and often number in the hundreds.7 Unlike polyps in the colon or small bowel, fundic gland polyps are a type of hamartoma. They are typically small (1 to 5 mm), sessile, and usually asymptomatic and are located in the fundus and body of the stomach.7 Adenomatous polyps of the stomach are occasionally found in FAP and AFAP.8 Gastric cancers arising from fundic gland polyps have been reported in FAP, although most are believed to arise from adenomatous polyps.8 Individuals with FAP have an 800–fold increased risk for desmoid tumors (aggressive fibromatoses), with a lifetime risk of 10% to 30%.9–11 Risk factors for desmoid tumors in FAP include a family history of desmoid tumors, APC mutations 3Ỳ to codon 1,399 (genotype–phenotype correlation), female sex, and previous abdominal surgery.10 Although desmoid tumors do not metastasize, they can be locally invasive, aggressive, and difficult to treat, resulting in significant morbidity and the second leading cause of mortality in FAP.12
The phenotypic spectrum of germ-line APC mutations also includes other benign findings such as osteomas, epidermoid cysts, fibromas, dental abnormalities, and congenital hypertrophy of the retinal pigment epithelium (CHRPE). In addition, there are increased risks for other cancers, including those of the pancreas, thyroid, bile duct, brain (typically medulloblastoma), and liver (specifically hepatoblastoma).6
Management
Without treatment, CRC is inevitable in FAP. However, with early screening and polypectomies, in addition to prophylactic colectomies after polyps become too difficult to manage endoscopically, most CRCs can be prevented in AFAP and FAP. In FAP, annual colonoscopies or flexible sigmoidoscopies are recommended starting around the age of 10 years.13 In AFAP, screening begins in the late teenage years, and colonoscopies, rather than sigmoidoscopies, are necessary because of proximally located polyps.13 Colectomies can sometimes be avoided in AFAP, which is not the case for individuals with FAP. After polyps become too numerous (usually >20 to 30 polyps) to manage endoscopically or when adenomas with advanced histology are identified, a prophylactic colectomy is advised.13 A proctocolectomy with an ileal pouch anal anastomosis is the standard surgery in FAP, whereas a total colectomy with ileorectal anastomosis is often the preferred approach with AFAP or in FAP cases with limited rectal involvement.13,14 Continued screening of the remaining rectum or ileal pouch is still necessary.13
Recently, it has been shown that duodenal cancer detected through surveillance improves survival compared with individuals presenting because of symptoms.15 The NCCN currently recommends the consideration of an esophagogastroduodenoscopy (EGD) with a side-viewing examination beginning around the age of 25 years for duodenal cancer surveillance.13 The extent of duodenal polyps, as defined by the Spigelman staging criteria, is used to determine the EGD follow-up interval.13 Additional considerations for the management in individuals with germ-line APC mutations are outlined and updated annually by the NCCN (www.nccn.org).
Genetic Testing and Counseling
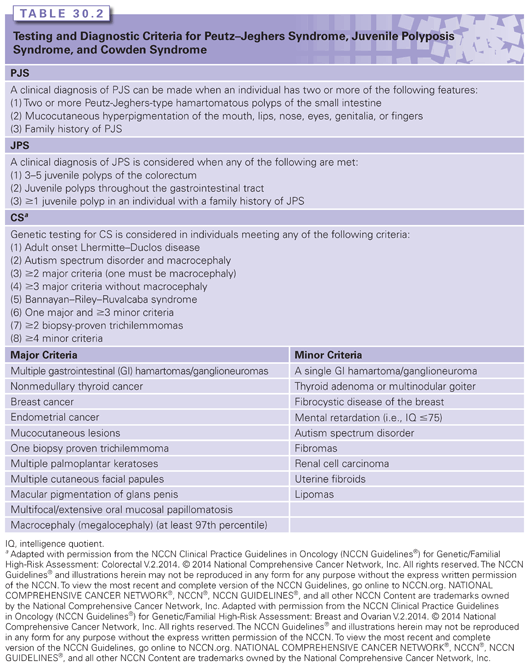
A clinical diagnosis of FAP is considered when at least 100 colorectal adenomatous polyps are detected by the 2nd or 3rd decade of life.6 Genetic testing of APC is still recommended to clarify extracolonic cancer risks and to help determine FAP status in relatives. Genetic testing has also been shown to be cost-effective,16 although it is unlikely to change colon management for cases with extensive adenomatous polyposis.
Given the phenotypic variability, a consensus as to what constitutes a diagnosis of AFAP has not been reached. The NCCN currently recommends that individuals with greater than 10 cumulative colorectal adenomas be referred for genetic counseling and the consideration of genetic testing.13 The identification of an APC mutation in these less severe polyp cases confirms a diagnosis of AFAP. It is also noteworthy that individuals with 100 or more adenomatous polyps may have AFAP if polyp development occurs at a later age (typically after 40 years).
Differentiating among FAP, AFAP, and other colonic polyposis conditions is not always straightforward. Family history, which is consistent with an autosomal-dominant mode of inheritance, is suggestive of FAP/AFAP and increases the likelihood of finding an APC mutation.6 However, 10% to 30% of probands with germ-line APC mutations are de novo (new mutation) cases, and consequently, their parents are unaffected.6,17 In addition, it is not uncommon for individuals with AFAP to have less than 10 cumulative adenomatous polyps.4 In patients with fewer polyps, it is not clear whether genetic testing should be performed.13 However, it is important that these individuals be closely followed up, and if multiple adenomas continue to develop, genetic testing should be reconsidered.
Unlike what is found in some of the other conditions described in this review, hyperplastic or hamartomatous colon polyps are not known to be associated with FAP/AFAP. Therefore, if multiple hyperplastic or hamartomatous colon polyps are found in an individual, a genetic testing of APC is unlikely to be informative. Other features associated with APC mutations that may assist with making a diagnosis of AFAP or FAP include fundic gland polyposis, duodenal adenomatous polyps, osteomas, CHRPE, desmoid tumors, and hepatoblastoma.6
MUTYH-Associated Polyposis
As the name implies, MAP is a colonic polyposis condition caused by germ-line mutations in the MUTYH gene. Contrary to the other conditions described in this review, MAP is inherited in an autosomal-recessive pattern. In 2002, Al-Tassan et al.18 were the first to describe a family with biallelic (homozygous or compound heterozygous) mutations in MUTYH, which is part of the base excision repair system. In this family, three siblings had CRC and/or multiple colorectal adenomas, but no detectable mutations in APC.18 All three of the affected siblings were found to have compound heterozygous mutations in MUTYH, whereas the other four unaffected siblings did not.18
It is now widely accepted that MAP is associated with a significant increased risk for multiple colorectal adenomas and cancer. Whether monoallelic MUTYH carriers have a modest increase in risk of CRC is debatable.19 Monoallelic mutations in MUTYH are found in 1% to 2% of the general population, whereas biallelic mutations account for less than 1% of all CRCs.20
Colonic Phenotype
There are a number of similarities between the colonic phenotype of MAP and AFAP, including the average number, proximal distribution, and young age at onset of adenomas and cancers.4,19 MUTYH-associated polyposis is associated with a 28-fold increased risk of CRC, with a penetrance of 19% by the age of 50 years, 43% by 60 years, and 80% by 70 years.19,21 Although the risk of CRC has been reported to be as high as 100%,22 the actual penetrance is likely to be incomplete and similar to that of AFAP. The total number of polyps in MAP is also highly variable, with some individuals developing CRC without polyps, whereas others have more than 500 colorectal polyps.23 Typically, affected individuals have between 10 and 100 polyps.23
Adenomas are the predominant polyp type seen not only in AFAP and FAP, but also in MAP. Unlike individuals with germ-line APC
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree