TUMOR SCREENING
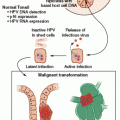
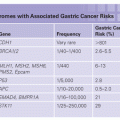
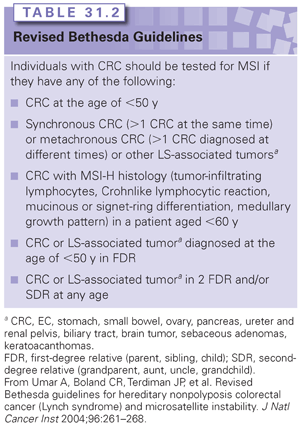
Microsatellites are pieces of DNA sequence where a single nucleotide or group of nucleotides is repeated multiple times. In general, the number of repeated nucleotide sequences should remain the same within a person’s cells, but when this number of repeats differs in one or two alleles, MSI is present.21 In the case of LS, five microsatellite markers are used as the standard with which to measure MSI, and a tumor is considered to have a high level of MSI (MSI-H) if at least 40% of the markers are unstable.22 Nearly all LS-associated tumors display MSI, but the presence of MSI is not diagnostic of LS, given that approximately 10% to 15% of all CRCs, in general, also display MSI.23,24 MSI testing, however, can be used as a screening tool to help identify individuals for whom germ-line genetic testing for mutations in MLH1, MSH2, MSH6, and/or PMS2 is indicated. A clinical diagnosis of LS can be made if a person has an MSI-H CRC and meets the Amsterdam II criteria (see Table 31.1).
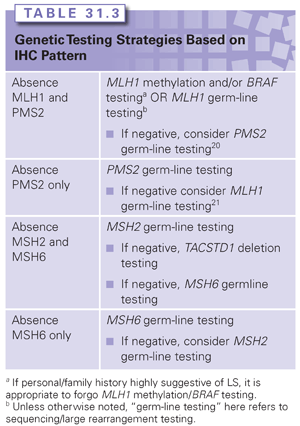
Immunohistochemistry can be used to determine the presence or absence of MMR proteins in a tumor specimen and is another available screening test for LS. The absence of one or more MMR proteins in tumor tissue indicates dysfunction of the corresponding MMR gene, but additional analyses are required to determine if the dysfunction is germ line or somatic in nature. The benefit of performing IHC staining over MSI testing is that results from IHC staining can direct the approach to genetic testing. For instance, if a patient has CRC that demonstrates an absence of MSH2 and MSH6 proteins, testing for MSH2 with procession to testing for MSH6 if the MSH2 test results are negative is recommended. In the context of this IHC result, it is generally not necessary to test for mutations in MLH1 and/or PMS2. Strategies for genetic testing based on IHC results are included in Table 31.3.25,26 In comparing tumor screening strategies, using MSI and IHC staining together will identify the majority of LS cases. Because the sensitivity of either test is not 100%, using either test alone will leave 5% to 10% of LS cases undetected.27,28
Epigenetic events unrelated to LS can cause a tumor to demonstrate MSI and an absence of MLH1 and PMS2 proteins upon IHC staining. These results can often be attributed to hypermethylation of the MLH1 promoter and/or a somatic BRAF mutation (V600E). Several studies have shown that the V600E BRAF mutations are not associated with LS, and there have been very few exceptions.11,29 Both of these tests can be performed on CRC tissue to help determine whether germ-line genetic testing should be pursued, further streamlining the genetic testing process, but this should be interpreted in the context of clinical familial presentation because sensitivities and specificities for these tests are not 100%. It is important to note that it is possible but rare to have inherited MLH1 promoter hypermethylation.30
Similar approaches to screening EC with MSI and/or IHC staining are appropriate. However, because somatic BRAF mutations are uncommon in ECs, BRAF testing is not an appropriate test for ECs that are MSI-H and/or MLH1- and PMS2-protein deficient.31 There are less data to support MSI and/or IHC testing using other LS-associated tumor tissue, but many reports suggest that it is at least feasible in the absence of additional testing options.32
In 2009, the Evaluation of Genomic Application in Practice and Prevention Working Group recommended that individuals with newly diagnosed CRCs be offered genetic testing for LS. Although the Evaluation of Genomic Application in Practice and Prevention Working Group did not specify the best approach for genetic testing, performing a tumor analysis with IHC staining allows for more targeted genetic testing and was considered to be an acceptable strategy.33 In addition, multiple reports have shown that tumor screening with IHC staining is a cost-effective strategy for identifying LS in the CRC patient population.34,35 Ladabaum et al.35 compared the differences among multiple testing strategies with regard to effect of life-years, cancer morbidity and mortality, and cost. They concluded that IHC staining with inclusion of BRAF gene testing if the MLH1 protein was absent was the preferred method of identifying LS among CRC patients.35 The effectiveness of screening for LS in these reports has been dependent on the ability to test family members of the initially diagnosed LS patient; therefore, genetic counseling and dissemination of information to relatives of probands are crucial for an effective diagnosis and the prevention of cancer.
GENETIC TESTING
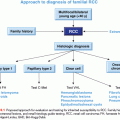
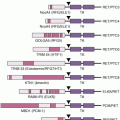
In many situations, as mentioned previously, tumor screening tests are performed before germ-line genetic testing, but there are situations where this is not possible or desired (e.g., sufficient tumor tissue is unavailable, all individuals affected with cancer in a family are deceased). In the absence of IHC results to direct genetic testing, germ-line testing is typically done in a stepwise fashion beginning with MLH1 and MSH2, which account for 32% and 38% of mutations, respectively.9 If no mutations are identified, testing for mutations in MSH6 and PMS2
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree