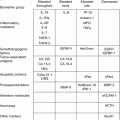
Syndrome
Manifestations
Inheritance
Gene(s)
Reference
Hereditary breast and ovarian cancer
Early-onset breast or ovarian cancer, multiple primary cancers, cancer in several generations, male breast cancer
AD
BRCA1
[19]
BRCA2
[20]
Hereditary nonpolyposis
Colon cancer, uterine cancer, ovarian cancer, stomach cancer, cancer in several generations
AD
MLH1
[21]
Colon cancer (HNPCC; aka Lynch syndrome)
MSH2
[22]
MSH6
[23]
PMS2
[24]
EPCAM
[25]
Familial pancreatic cancer
Pancreatic cancer, breast cancer, ovarian cancer, cancer in several generations
AD
PALB2
[26]
Fanconi anemia
Skeletal defects, anemia, bone marrow failure, eye or ear defects, congenital heart defects, renal defects
AR
BARD1
[27]
BRIP1
[28]
MRE11A
[29]
NBN
[30]
RAD50
[31]
RAD51C
[32]
Li-Fraumeni syndrome
Breast, brain, adrenal, sarcomas, leukemia
AD
TP53
[33]
Peutz-Jeghers syndrome
Multiple gastrointestinal hamartomatous polyps; melanocytic macules of the lips, buccal mucosa, and digits; GI cancers; breast, lung, thyroid, pancreatic, uterine, ovarian sex cord tumors
AD
STK11
[34]
CHEK2
Breast cancer; may resemble Li-Fraumeni syndrome phenotype
AD
CHEK2
[27]
Cowden syndrome
Gastrointestinal hamartomatous polyps; breast, ovarian, uterine, cervical, thyroid, bladder cancers; mucocutaneous lesions
AD
PTEN
[35]
Bannayan-Ruvalcaba- Riley syndrome
Macrocephaly, angiomatosis, lipomatosis, childhood onset (cf. Cowden)
AD
PTEN
[36]
Although clinical testing is available for these genes, there seems to be no significantly increased risk for ovarian cancer associated with them, although there is for other gynecologic malignancies:
Ataxia-telangiectasia
Cerebellar ataxia, telangiectases, immune system defects, breast cancer in heterozygous relatives of probands (not ovarian cancer)
AR
ATM
[37]
Hereditary diffuse gastric cancer
Gastric cancer
AD
CDH1
[38]
Familial adenomatous polyposis, Type 2
Multiple colorectal adenomas
AD
MUTYH
[39]
Massively parallel sequencing (also called next-generation sequencing) is a high-throughput group of technologies that allows rapid simultaneous determination of single-base substitutions, small deletions and insertions, and large gene rearrangements in several genes. Recent studies of inherited mutations in breast and ovarian cancer [40] and ovarian, fallopian tube, and peritoneal carcinoma [41] have demonstrated the utility of the technique and identified several genes not previously suspected of being involved in ovarian cancer. In the latter study, 360 women undergoing primary surgery (273 with ovarian carcinomas, 48 with peritoneal carcinomas, 31 with fallopian tube carcinomas, and 8 with synchronous ovarian and endometrial carcinomas) had their germline DNA analyzed for several genes. Deleterious mutations were found in 24 % of the subjects, 18 % in BRCA1 and BRCA2, and 6 % in several other genes (BARD1, BRIP1, CHEK2, MRE11A, MSH6, NBN, PALB2, RAD50, RAD51C, or TP53). In the women with inherited mutations, over 30 % had no family history of breast or ovarian cancer, and more than 35 % were over 60 years old at the time of diagnosis. The authors stated that massively parallel sequencing of several genes simultaneously, as compared to sequential sequencing of individual genes, could lower the cost while increasing the identification of patients and families with germline mutations.
Recently, testing for several genes using next-generation sequencing has become available on a clinical basis. Since mutations in each of these genes are much less prevalent than mutations in BRCA1 or BRCA2, testing these genes should usually be considered a second-tier option for most patients, reserved for patients who do not have a detectable mutation in BRCA1 or BRCA2. One commercially available panel offers testing for ATM, BARD1, BRIP1, MRE11A, NBN, RAD50, RAD51C, CHEK2, CDH1, EPCAM, MLH1, MSH2, MSH6, PMS2, MUTYH, PALB2, PTEN, STK11, and TP53. It should be noted that some of these genes are associated with an increased risk of breast or uterine cancer, not ovarian cancer, but testing for individual genes in this panel is also available.
Management of High-Risk Patients
Identification of a BRCA1 or BRCA2 mutation warrants more intensive surveillance and more aggressive management due to the increased risk for a cancer that typically causes few symptoms in the early stages. There is evidence that risk-reducing oophorectomy reduces the incidence of ovarian cancer substantially in mutation carriers [42–44], although the risk of peritoneal cancer remains at about 2 % after surgery [45, 46].
For women with a mutation, the NCCN has specific recommendations [47]. For prevention or early detection of breast or ovarian cancer, these recommendations include: (1) breast self-exam and education starting at age 18; (2) clinical breast exam every 6–12 months starting at age 25; (3) annual mammogram and breast magnetic resonance imaging screening starting at age 25 or earlier based on the earliest age of onset of breast cancer in the family; (4) a discussion of prophylactic mastectomy; (5) risk-reducing salpingo-oophorectomy, ideally between ages 35 and 40 and after childbearing is complete or earlier based on the earliest age of onset of ovarian cancer in the family; (6) for those not electing risk-reducing salpingo-oophorectomy, the recommendation is transvaginal ultrasound, preferably during days 1–10 of menstrual cycle in premenopausal women, plus cancer antigen 125 (CA-125) measurements (preferably after day 5 of the menstrual cycle in premenopausal woman) every 6 months starting at age 35 or 5–10 years before the earliest age of first diagnosis of ovarian cancer in the family; (7) consideration of chemoprevention (e.g., tamoxifen, raloxifene, exemestane); and (8) considering investigational imaging and screening studies when available in the context of a clinical trial. Regarding the salpingo-oophorectomy recommendation, NCCN Guidelines state: “Counseling includes a discussion of reproductive desires, extent of cancer risk, degree of protection for breast and ovarian cancer, management of menopausal symptoms, possible short term hormone replacement therapy (HRT) to a recommended maximum age of natural menopause, and related medical issues” [5]. There is no evidence that transvaginal ultrasound and serum CA-125 measurements reduce ovarian cancer mortality [48, 49].
Family Planning
If a mutation is identified in any man or woman, there is a 50 % chance of passing on that mutation to any child they have. If the potential parent does not want to pass on the mutation to the next generation, prenatal diagnosis and preimplantation genetic diagnosis are technically possibly but rarely requested in clinical practice and beyond the scope of this chapter.
Conclusion
Ovarian cancer remains a diagnosis with a grim prognosis, mainly due to the late stage at which is it usually diagnosed. For a group of patients, however, there is hope for an improved outcome due to the recognition of some germline mutations that can identify those with an increased risk for developing the disease. There is some evidence that earlier and more frequent surveillance of these patients may result in (1) prevention of the disease, mainly through prophylactic oophorectomies and (2) decreased morbidity and mortality due to earlier diagnosis and treatment.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree