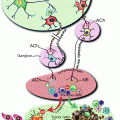
Fig. 15.1
Phenotypes of the alcohol dehydrogenase-1B*1/*1 (ADH1B*1/*1) and aldehyde dehydrogenase-2*1/*2 (ALDH2*1/*2) genotypes and their relationships to ethanol and acetaldehyde exposure and accumulation
15.3 ALDH2 Genotype and Squamous Cell Neoplasia in the UADT
In 1996, we first reported that being heterozygous for inactive ALDH2 is a strong risk factor for esophageal cancer in daily drinkers and alcoholics [12]. Since then, epidemiological studies have almost consistently demonstrated a strong association between the ALDH2*1/*2 genotype and the risk of UADT cancer in East-Asian drinkers [13]. A meta-analysis of Asian case–control studies of esophageal cancer showed that the ALDH2*1/*2-associated odds ratio (95 % confidence interval) [OR (95 % CI)] for esophageal cancer was 3.12 (1.95–5.02) in moderate drinkers, 5.64 (1.57–20.25) in ex-drinkers, and 7.12 (4.67–10.86) in heavy drinkers [14]. The ALDH2*1/*2 genotype has been found to be a very strong risk factor for esophageal cancer among Taiwan Chinese and Japanese drinkers (OR = 4.74–6.21 in moderate drinkers and 9.21–9.75 in heavy drinkers), but the OR associated with the ALDH2*1/*2 genotype in the high incidence regions of esophageal cancer of Mainland China was not so high (OR = 1.98 in moderate drinkers and 1.31 in heavy drinkers). The meta-analysis confirmed that being homozygous for the inactive allele, i.e., having the ALDH2*2/*2 genotype greatly increased the risk of esophageal cancer in drinkers, and that the heterozygous genotype, i.e., ALDH2*1/*2 increased the risk of esophageal cancer in a similar manner in both men and women. A meta-analysis of 6 Japanese case–control studies showed that the ALDH2*1/*2-associated risk for head and neck cancer was also greater in heavy drinkers [OR (95 % CI) = 3.57 (1.21–2.77)] than in moderate drinkers [OR (95 % CI) = 1.68 (1.22–2.27)] [15].
The United States National Cancer Institute’s SEER Program reported synchronous multiple cancers and metachronous multiple cancers in only 2 % and 3 %, respectively, of esophageal cancer patients during the 1973–2003 period [16]. The prevalence of multiple organ cancers among esophageal cancer patients treated at the National Cancer Center of Japan increased at an alarming rate between 1969 and 1996 from 6.3 % in 1969–1980, to 22.2 % in 1981–1991, and to 39.0 % in 1992–1996 [17], and the most frequent sites of the other cancers were the head and neck and stomach. The increased proportion of multiple cancers in Japanese esophageal cancer patients may partly explained by a dramatic increase in the proportion of ALDH2*1/*2 heterozygotes among Japanese heavy drinkers during the same period [10]. The ALDH2*1/*2 genotype has consistently been demonstrated to be a strong determinant of the risk of synchronous and metachronous SCC of the UADT in Japanese drinkers [13].
The ORs (95 % CI) associated with the ALDH2*1/*2 genotype in Japanese alcoholics has been found to increase for the very early stages of the esophageal neoplasia, from 2.88 (1.81–4.57) for low-grade intraepithelial neoplasia, to 5.14 (2.87–9.19) for high-grade intraepithelial neoplasia, and to 4.07 (1.97–8.40) for invasive SCC [18]. The presence of multiple esophageal iodine-unstained lesions or a large esophageal dysplasia has been found to be a strong predictor of the development of multiple cancers in the UADT in Japanese [13], and to be associated with the ALDH2*1/*2 genotype [18], p53 alteration [19], and telomere shortening in the esophagus [20] in Japanese alcoholics. Thus, acetaldehyde, which is an established human carcinogen, plays a critical role in the multicentric development of neoplasia throughout the UADT.
15.4 The Simple Flushing Questionnaire
The discovery of ALDH2-associated cancer susceptibility emphasizes the importance of developing screening tests for inactive ALDH2 based on alcohol flushing. We devised a flushing questionnaire to identify person with inactive ALDH2 (Fig. 15.2) [21, 22]. The simple flushing questionnaire consists of two questions: (A) Do you have a tendency to flush in the face immediately after drinking a glass (≈180 mL) of beer? (B) Did you have a tendency to flush in the face immediately after drinking a glass of beer during the first to second year after you started drinking? The results are used to classify the subjects as a current flusher, former flusher, or never flusher. When current flushers or former flushers were assumed to have inactive ALDH2, the simple flushing questionnaire had 90 % sensitivity and 88 % specificity when evaluated in 610 Japanese men 40 years of age or older [22] and 88 % sensitivity and 92 % specificity when evaluated in 381 Japanese women 40 years of age or older [23]. The individuals’ risks of UADT cancer estimated on the basis of the replies to the flushing questionnaire were slightly lower than but essentially comparable to their risks estimated on the basis of ALDH2 genotyping [21–23].


Fig. 15.2
The simple flushing questionnaire to identify persons with inactive ALDH2. Sensitivity and specificity are both approximately 90 % in Japanese 40 years of age and over, regardless of gender
15.5 Mass-Screening for SCC of the UADT by Means of Health-Risk Appraisal Models
Based on the results of a case–control study [24], we devised health-risk appraisal (HRA) models for esophageal cancer that include ALDH2 genotype or alcohol flushing [25]. The total risk score is calculated by adding the scores A–E (Fig. 15.3). If a person’s risk score is 11 or more according to the HRA-Flushing model, that person’s risk of esophageal cancer is in the top 10 % of the study population. A cross-validation study predicted that approximately 60 % of the esophageal and hypopharyngeal SCCs in the entire population could be detected by examining only people whose risk scores were in the top 10 % in the HRA models. Follow-up endoscopy with esophageal iodine staining (median follow-up period: 5.0 years) was performed on 404 cancer-free controls and resulted in the diagnosis of six esophageal SCCs and two pharyngeal SCCs [26]. The risk scores of six of these eight cancer patients at baseline were in the top 10 % according to the HRA-Flushing model. The cancer detection rate per 100 person–years in the top 10 % risk group of the cancer-free controls was 2.3 for esophageal cancer and 3.5 for esophageal or pharyngeal cancer. We applied this HRA-Flushing questionnaire to endoscopic mass-screening programs of 2,221 Japanese men during 2008 and 2009 at five cancer screening facilities, and esophageal cancer was diagnosed in 19 persons as a result [27]. The HRA-Flushing score of 5 % of the examinees was 11 or greater, and esophageal cancer was detected in 4.3 % of them, as opposed to in 0.7 % of the other examinees. A receiver operating characteristic curve analysis showed that when we used an cutoff point of an HRA score of ≥9 in the 50–69 age group and of ≥8 in the 70–89 age group to select individuals with a high risk for esophageal cancer, the sensitivity and false-positive rate was 52.6 % and 15.2 %, respectively, and cancer was detected in 2.91 % of the examinees in the high-risk group, as opposed to 0.48 % in the other group. Although the cutoff values for high-risk groups should be changed to achieve better performance of the HRA model according to the population targeted, these figures encouraged using our questionnaire to screen larger populations of Japanese men.
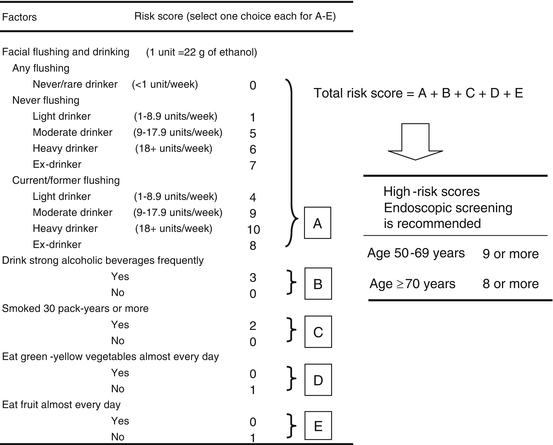

Fig. 15.3
Health-risk appraisal model for esophageal cancer combined with the simple flushing questionnaire. The questionnaire enables makes it possible for people to easily determine their risk of esophageal cancer, and public awareness campaigns that use the questionnaire will help persuade high-risk persons to undergo endoscopic screening or enable them to change their lifestyle to prevent esophageal cancer
Our simple questionnaire makes it possible for many people to identify their risk of UADT cancer very easily, and use of the questionnaire in public awareness campaigns will help persuade high-risk persons to undergo endoscopic screening or enable them to change their lifestyle to prevent UADT cancer. Our HRA model that includes ALDH2 genotype yielded a slightly better positive predictive value [25] and a slightly higher cancer detection rate [26] than the HRA-Flushing model. Genotyping ALDH2 would entail an initial cost, but genotyping needs to be performed only once in a lifetime, the data are always available, and the unit cost would be greatly discounted if a huge number of samples are analyzed.
15.6 ADH1B Genotype, Alcohol Metabolism, and SCC of the UADT
Alcohol dehydrogenase-1B (ADH1B, previously called ADH2) also has a functional genetic polymorphism (rs1229984; Fig. 15.1). The ADH1B*1/*1 genotype, which results in expression of slow-metabolizing ADH1B, is prevalent in Caucasians (present in approximately 90 %), but present in only a small fraction of East Asians (e.g., 7 % of Japanese) [6]. The presence of slow-metabolizing ADH1B is a stronger risk factor for alcoholism and SCC of the UADT in East Asians than the presence of the fast-metabolizing ADH1B because of having the ADH1B*2 allele. Approximately 30 % of Japanese alcoholics have the slow-metabolizing ADH1B, and the slow-metabolizing ADH1B has been found to be more frequent in the younger generations of Japanese alcoholics, probably because the ADH1B*1/*1 genotype accelerates the progression of alcohol dependence [11].
Alcohol challenge tests have failed to demonstrate any associations between ADH1B genotype and the blood ethanol or acetaldehyde concentrations after ingestion of small to moderate doses of ethanol [13]. However, the slow-metabolizing ADH1B has an approximately 40 times lower Vmax in vitro than the fast-metabolizing ADH1B [28], and an experiment in which a clamping technique and intravenous alcohol infusion were used showed a modestly but significantly lower ethanol elimination rate (11–18 %) among Jews with the slow-metabolizing ADH1B than with the fast-metabolizing ADH1B [29]. At the time of their first visit to our Addiction Center, we evaluated associations between ADH1B and ALDH2 genotypes and the blood ethanol levels of 805 Japanese alcoholic men in the morning after drinking within the previous 34 h [30]. The results showed no significant differences in age-adjusted usual alcohol consumption according to ADH1B or ALDH2 genotypes. Higher blood ethanol levels persisted for longer periods in the group with the slow-metabolizing ADH1B who were ADH1B*1/*1 carriers (n = 246) than in the group with the fast-metabolizing ADH1B who were ADH1B*2 carriers (n = 559), and blood ethanol levels ≥0.3 mg/mL (criterion for drunk driving according to Japanese law) after a 12.1–18-h interval since the last drink were observed in a significantly higher proportion of the ADH1B*1/*1 carriers than of the ADH1B*2 carriers (40 % vs. 14–17 %, p < 0.0001). Multivariate analyses showed that the ethanol levels were 0.500 mg/mL higher in the group with the ADH1B*1*1 genotype, and the OR (95 % CI) for an ethanol level ≥0.3 mg/mL in the presence of the ADH1B*1/*1 genotype was 3.44 (2.34–5.04). There were no significant differences in blood ethanol levels according to ALDH2 genotype.
We evaluated associations between ADH1B and ALDH2 genotypes and the body weight and BMI of 1,301 Japanese alcoholic men on the day of their first visit [31]. There were no significant differences in usual caloric intake in the form of alcoholic beverages according to ADH1B genotype in any of the age brackets, but the presence of the slow-metabolizing ADH1B was more strongly associated with weight gain in all age brackets. This result links the slower ethanol elimination by the ADH1B*1*/*1 alcoholics with their more efficient utilization of ethanol as an energy source. No effects of ALDH2 genotype on body weight or BMI were observed.
In a study in which we simultaneously measured the blood and salivary ethanol and acetaldehyde levels of Japanese alcoholics in the morning on the day of their first visit [32, 33], we found that ethanol and acetaldehyde remained in the blood and saliva for much longer periods and at much higher levels in the group with the slow-metabolizing ADH1B than in the group with the fast-metabolizing ADH1B, even after adjusting for age, body weight, the amount of alcohol consumed, and interval since the previous drink. Chronic heavy drinking by alcoholics may amplify the modest effect of ADH1B*1/*1 on ethanol metabolism and lead to clear prolongation of the presence of ethanol in the body, including in the UADT. The blood and salivary ethanol levels of the subjects were similar, but the acetaldehyde levels in their saliva were much higher than in their blood because of acetaldehyde production by oral microorganisms. [32, 33]
ADH1B genotype markedly affects alcohol flushing [22, 34]. Alcohol flushing in fast-metabolizing ADH1B carriers is triggered by a rapid initial rise in the blood acetaldehyde concentration, whereas the slow initial rise in the blood acetaldehyde in slow-metabolizing ADH1B carriers may weaken the alcohol flushing [22, 34]. The results of our flushing questionnaire showed that despite the presence of inactive ALDH2 in heterozygotes, 25 % of the slow-metabolizing ADH1B carriers were never flushers and 38 % were former flushers, and thus there was a clear association between alcohol consumption by inactive ALDH2 heterozygotes and their facial flushing categories [22].
The above findings provide clues as to why slow-metabolizing ADH1B increases the risk of both alcoholism and UADT cancer. First, slow-metabolizing ADH1B diminishes the intensity of facial flushing, thereby accounting for the greater susceptibility to heavy drinking. Second, chronic heavy drinking amplifies the modest effect of slow-metabolizing ADH1B on ethanol elimination, which leads to much longer exposure to ethanol and, in turn, results in increasing the risk of developing alcoholism. When ethanol lingers in the body, the UADT is exposed to high levels of acetaldehyde as a result of acetaldehyde production in saliva, and that creates a condition that increases the risk of UADT cancer.
15.7 The ADH1B*1/*1 and ALDH2*1/*2 Genotype Combination and SCC in the UADT
The risk of SCC of the head and neck and esophagus of Japanese and Taiwanese drinkers has been found to be extremely increased in a multiplicative fashion by the combination of slow-metabolizing ADH1B in the ADH1B*1/*1 genotype and inactive ALDH2 in the ALDH2*1/*2 genotype (OR = 22–122 for esophageal SCC) [13, 18, 24, 35–37]. In Japanese alcoholics, the OR (95 % CI) with the ADH1B*1/*1 and ALDH2*1/*2 genotype combination has been found to increase for the very early stages of the esophageal neoplasia, from 4.53 (2.17–9.47) for low-grade intraepithelial neoplasia, to 10.4 (4.34–24.7) for high-grade intraepithelial neoplasia, and 21.7 (7.96–59.3) for invasive SCC [18]. When the two genotypes were combined with other risk factors, based on the multivariate OR for each risk factor the ORs (95 % CI) of Japanese for esophageal SCC increased enormously, by 248 times when combined with consumption of 198–395 g ethanol/week and by 414 times when combined with consumption of ≥396 g ethanol/week [24], in Taiwanese by 382 (47–3,085) times when combined with consumption of >30 g ethanol/day [35], and in Japanese by 189 (95–377) times when combined with both consumption of >96.5 g ethanol/week and smoking [36] and by 357 (105–1,210) times when combined with both drinking and smoking [37].
15.8 ADH1B and ALDH2 Genotype and Liver Disease in Japanese Alcoholic Men
Contrary to results of investigations of the relationships between the ADH1B and ALDH2 genotypes and susceptibility to UADT cancer, a cross-sectional survey of 1,902 Japanese alcoholic men showed that liver cirrhosis was associated with the presence of the ADH1B*2 allele and ALDH2*1/*1 genotype [38]. When non-cirrhotic patients with no or only mild liver fibrosis as controls, the results showed that the OR (95 % CI) of the ADH1B*2 allele increased according to the severity of their liver disease, from 1.67 (1.32–2.11) in the non-cirrhotic group with liver fibrosis, to 1.81 (1.24–2.63) in the Child-Pugh class A cirrhosis group, 2.97 (1.79–4.93) in the Child-Pugh class B cirrhosis group, and 4.32 (1.48–12.6) in the Child-Pugh class C cirrhosis group. Since age-adjusted daily alcohol consumption did not differ according to ADH1B/ALDH2 genotypes in the alcoholics, their ADH1B/ALDH2-associated increase in risk of liver disease cannot be explained by the levels of ethanol and acetaldehyde exposure.
15.9 Gastric Cancer in Japanese Alcoholic Men
The lifetime drinking profiles of Japanese alcoholic men have shown that gastrectomy increases susceptibility to alcoholism [39]. Gastrectomy results in swift passage of ethanol from the small intestine into the systemic circulation. Because of this dynamic change in ethanol delivery, overshoot of blood ethanol levels and subsequent high ethanol exposure have been observed after gastrectomy [40], and they lead to rapid development of alcohol dependence. A large survey of 4,879 Japanese alcoholic men demonstrated that a high proportion of them had a history of gastrectomy, although the proportion decreased from 13.3 to 7.8 % during the 1996–2010 period [11]. Many alcoholic men with a history of gastrectomy had changed their drinking pattern after the gastrectomy and had became alcoholics after a shorter period of heavy drinking and after a lower cumulative alcohol intake than alcoholics with no history of gastrectomy [39]. There were more frequent blackouts in the gastrectomy group, and that may have reflected the sharper rise in their blood ethanol level. Since a history of gastrectomy increases the risk of alcohol dependence, this acquired risk factor increases susceptibility to alcohol dependence in the absence of the alcoholism-susceptibility genotype ADH1B*1/*1, and that explains the lower frequency of ADH1B*1/*1 that was found in a gastrectomy group of Japanese alcoholic men than in a non-gastrectomy group [11].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree