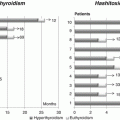
Fig. 19.1
Generation of functional thyroid follicles from pluripotent stem cells. (a) Schematic diagram of the thyroid follicle differentiation protocol from ESCs. (b) Expression of endogenous Nkx2-1 and Pax8, Foxe1, Tshr, Slc5a5, Tg and Tpo at day 22 in cells differentiated after Doxycycline-mediated induction of Nkx2-1–Pax8 and subsequent treatment with rhTSH. Relative expression of each transcript is shown as fold change compared to untreated cells (baseline) at day 22 as mean ± s.e.m. (n = 6). Unpaired t-test was used for statistical analysis. *P < 0.05, **P < 0.01, ***P < 0.001. (c–i) Immunostaining at the end of the differentiation (day 22) for NKX2-1 and PAX8 (c), NKX2-1 and FOXE1 (d), NKX2-1 and TG (e, h), NKX2-1 and NIS (f, g), NKX2-1 and iodinated TG (TG-I) (i). (j) Histogram showing the organification percentage of iodine-125 at day 22 in ESC-derived thyroid follicles; undifferentiated cells column refers to cells at day 22 differentiated without Doxycycline and rhTSH (Modified from Antonica et al. [50])
At the end of the differentiation, cells differentiated after induction of both transcription factors and subsequent rhTSH treatment show at transcriptional level the up-regulation of the four transcription factors (Nkx2-1, Pax8, Hhex and Foxe1), known to play a pivotal role during thyroid development, and of the genes encoding for the protein important for thyroid hormone synthesis such as TG, TPO, TSHR and NIS (Fig. 19.1b). Immunofluorescence analysis of the cells shows clusters of cells co-expressing thyroid markers NKX2-1, PAX8, FOXE1, TG and NIS (Fig. 19.1c–f). Detailed morphological characterization showed that ESC-derived thyroid cells were capable to organize into follicular structures, confirmed by polarization of NIS at the basolateral membrane (Fig. 19.1g) and accumulation of TG in the luminal compartment (Fig. 19.1h), while functional analysis highlighted the capacity of these cells to metabolize iodide (Fig. 19.1i, j) [50]. More interestingly, confirming their functionality, ESC-derived thyroid follicles can be transplanted into hypothyroid mice (Fig. 19.2a). The exogenous tissue, transplanted under the renal capsule, can perfectly integrate into the host, as shown by the presence of follicular epithelium (Fig. 19.2b, c) histologically positive to the transcription factors (NKX2-1, PAX8 and FOXE1) [50], functional thyroid markers NIS (Fig. 19.2d) and TG (Fig. 19.2e) and finally T4 (Fig. 19.2f) and more interestingly, the formation of angiofollicular units shown as NKX2-1-forming follicles surrounded by endothelial cells forming vessels (Fig. 19.2g). The correct integration, functionality and formation of a vascular network around the transplanted ESC-derived thyroid follicles lead to the restoring of the normal serum level of thyroid hormones (Fig. 19.2h) together with the normalization of thyroid homeostasis, shown as a decreased serum level of TSH (Fig. 19.2i) and a symptomatic recovery from the hypothyroidism state shown as a normalization of the body temperature (Fig. 19.2j) [50].
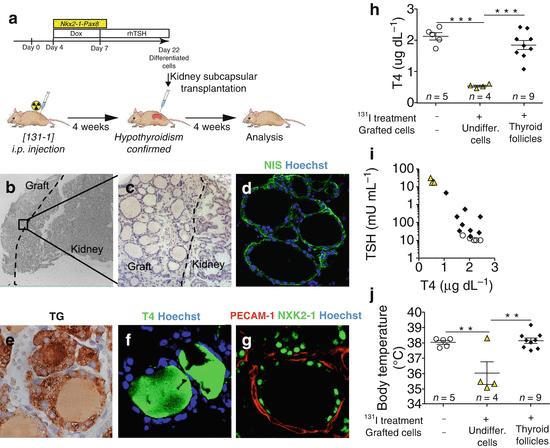

Fig. 19.2
Rescue of hypothyroidism in mice by transplantation of ESC-derived thyroid follicles. (a) Schematic diagram of protocol for ESC-derived thyroid follicles transplantation under the renal capsule of mice after radio-iodine ablation of thyroid. (b–g) Histological examination of kidney sections 4 weeks after grating. Hematoxylin-eosin staining shows the integration of the transplanted tissue in the cortical area of the host kidney (b) characterized by single cuboidal epithelium organization of transplantanted tissue (c). Immunofluorescence analysis confirms the expression of Nis (d) and Tg (e), production of T4 (f) and formation of vessels (PECAM-1) surrounding NKX2-1-forming follicles (g). (h) Total plasma T4 levels 4 weeks after transplantiation of ESC-derived thyroid follicles in iodine-131-treated mice. (i) Relationship between plasma TSH and T4 levels 4 weeks after grafting. (j) Body temperature measurements 4 weeks after grafting. In (h–j), open circles show iodine-131-untreated and ungrafted mice; yellow triangles show mice treated with iodine-131 and grafted with undifferentiated cells (cells cultured without Dox and rhTSH) and black diamonds show mice treated with iodine-131 and grafted with ESC-derived thyroid follicles. The values are shown as a dot plot (h, j) or scatter plot (i) and data are mean ± s.e.m. Tukey’s Multiple Comparison Test (h, j) was used for statistical analysis. **P < 0.01, ***P < 0.001 (Modified from Antonica et al. [50])
19.4 Embryonic Stem Cell as Model to Study Thyroid Embryogenesis and Modelling Congenital Hypothyroidism
The establishment of the first protocol of differentiation of pluripotent stem cells into functional thyroid cells has provided a novel and complementary system to study thyroid organogenesis. To date, very few studies have been performed in vivo in order to obtain a genome-wide expression profile of the thyroid cells at different developmental stages [56]. The in vitro model of thyroid organogenesis would be an alternative system to study and uncover new genes playing a role during the embryonic development of the gland. The unlimited source of biological material makes this model very useful to get access to the complete transcriptional profile. Moreover, the model represents also a valid system to study the function of genes (generate ESC mutant lines for specific genes and evaluate the effect during the in vitro differentiation) or signalling pathway hypothetically important for the commitment to thyroid fate or for the survival of thyroid cells and even their organization into follicles. Obviously, the system doesn’t seem to be suitable to study migration of thyroid cells, another aspect important for the correct development of the gland.
This approach would be interestingly translated from mice to humans in order to uncover new genetic causes of congenital hypothyroidism. During the last years, human embryonic stem cells and more interestingly induced pluripotent stem cells have been emerging as a very interesting tool for the in vitro modelling of human diseases.
In 2006, Shinya Yamanaka – Nobel Prize for medicine and physiology 2012 – showed that mouse fibroblasts could be reprogrammed into embryonic stem cell-like cells by the simultaneous ectopic expression of four genes [57]. These cells were called induced pluripotent stem cells (iPSCs). More interestingly, 1 year later they reported that a similar experimental approach was used to reprogram adult human fibroblasts into iPSCs [58]. Briefly, the co-expression of the four key transcription factors KLF4, SOX2, c–MYC and POU5F1 (also known as OCT4) successfully reprograms differentiated somatic cells back to a pluripotent state [58]. During the last 5 years, it has been shown how iPSCs can be differentiated into many different cell types, in similar ways as ESCs.
As it has been discussed, only few mutations in genes already known to be important for thyroid development have been found in CH patients with thyroid dysgenesis [59]. Several studies have been published during the last year demonstrating how iPSCs can be used in vitro for modelling genetic diseases [60].
The most reasonable approach would be to take advantage of the iPSC technology to derive pluripotent stem cells from fibroblasts of CH patients with thyroid dysgenesis. Applying the protocol of differentiation to CH patient-derived iPSCs might reveal differences at the transcriptional and morphological levels compared with healthy control-derived iPSC. This approach would lead to identify new genes playing an important role in normal and pathological thyroid development. Nevertheless, the selection of CH cases will be important as well as the good controls essential for the validation of the approach. Due to the fact that the in vitro model cannot be used for studying the migration of the gland during the foetal life, CH patients with thyroid ectopy should be excluded. Discordant monozygotic twins represent an interesting category of CH cases, and our model could help us to understand the genetic causes of a different thyroid phenotype in two ‘identical’ individuals. Our model might represent a good approach to investigate the causes of the differential thyroid organogenesis occurring in discordant monozygotic twins when mutations in coding or regulatory regions (exome sequencing and whole genome sequencing provide an additional tool to discover new mutations) lead to a differential expression of genes important for the development of the gland. Nevertheless, the model might not be a suitable model when epigenetic events occurring during early embryogenesis of the monozygotic twins are the cause of a differential development of the gland. Another interesting study would be the analysis of familial cases of CH. Exome sequencing of an entire family with CHTD (congenital hypothyroidism with thyroid dysgenesis)-affected members in combination with full transcriptional profiling of cells differentiated from healthy and CHTD-derived iPSCs would represent an interesting and valid approach to uncover new genes involved during thyroid embryogenesis. Identifying new genes will have a clinical impact increasing the list of genes that can be screened during pre-natal diagnosis.
Finally, it has been shown that ESC-derived thyroid follicles can be transplanted in vivo and restore thyroid homeostasis in mice affected by hypothyroidism. Those findings open new avenues towards conception of stem cell technology for the treatment of hypothyroidism. Nevertheless, I strongly believe we are still far from applying this approach for regenerative medicine. For example, genetic instability, due to culture conditions or the genetic engineering, together with the difficulty to obtain always homogeneous populations of differentiated cells are important pitfalls for regenerative medicine.
19.5 Conclusions
In this chapter, I discussed the first in vitro protocol of differentiation of pluripotent stem cells into functional thyroid follicles. This model enters in the large and steadily growing list of tissues that can be differentiated in 3D stem cell culture. This model would represent an additional and complementary model to study and characterize molecular and morphological events occurring in thyroid organogenesis. More interestingly, this ESC-based model can be used to uncover new genetic causes of thyroid dysgenesis in CH patients. In the far future, we are facing the idea to use the approach of curing severe hypothyroidism using transplantation of autologous ESC-derived thyroid tissue.
References
1.
Opitz R, Antonica F, Costagliola S (2013) New model systems to illuminate thyroid organogenesis. Part I: an update on the zebrafish toolbox. Eur Thyroid J 2:229–242PubMedCentralPubMedCrossRef
2.
Lazzaro D, Price M, de Felice M, Di Lauro R (1991) The transcription factor TTF-1 is expressed at the onset of thyroid and lung morphogenesis and in restricted regions of the foetal brain. Development 113:1093–1104PubMed
3.
Plachov D, Chowdhury K, Walther C, Simon D, Guenet JL, Gruss P (1990) Pax8, a murine paired box gene expressed in the developing excretory system and thyroid gland. Development 110:643–651PubMed
4.
5.
Dathan N, Parlato R, Rosica A, De Felice M, Di Lauro R (2002) Distribution of the titf2/foxe1 gene product is consistent with an important role in the development of foregut endoderm, palate, and hair. Dev Dyn Off Publ Am Assoc Anatomists 224:450–456
6.
Bogue CW, Ganea GR, Sturm E, Ianucci R, Jacobs HC (2000) Hex expression suggests a role in the development and function of organs derived from foregut endoderm. Dev Dyn Off Publ Am Assoc Anatomists 219:84–89
7.
Thomas PQ, Brown A, Beddington RS (1998) Hex: a homeobox gene revealing peri-implantation asymmetry in the mouse embryo and an early transient marker of endothelial cell precursors. Development 125:85–94PubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree