Fig. 18.1
Therapy-targeted pathways in lung cancer
Targeted therapy in lung cancer and the drugs that influence them. EGF, epidermal growth factor; TGF-α, transforming growth factor-α; EGFR, epidermal growth factor receptor; HER2/3, human epidermal growth factor receptor 2/3; PI3K, phosphatidylinositol-4,5-bisphosphate 3-kinase; SOS, son of sevenless guanine nucleotide exchange factor; GRB2, growth factor receptor-bound protein 2; PTEN, phosphatase and tensin homologue; mTOR, mammalian target of rapamycin; STAT 3/5, signal transducer and activator of transcription 3/5; EML4-ALK, echinoderm microtubule-associated protein-like 4-anaplastic lymphoma kinase; BRAF, B-Raf proto-oncogene, serine/threonine kinase; MEK, MAPK/Erk kinase; MAPK, mitogen-activated protein kinase
Table 18.1
Targeted therapy in non-small cell lung cancer (NSCLC)
Therapy type | Therapy agent | Type/class | Target | Evidence |
|---|---|---|---|---|
Anti-EGFR therapy | Gefitinib | Small molecule inhibitor | EGFR protein | |
Erlotinib | Small molecule inhibitor | EGFR protein | ||
Afatinib | Small molecule inhibitor | EGFR and other ERBB family members | ||
ALK inhibitors | Crizotinib | Small molecule inhibitor | EML4-ALK kinase activity | |
Multikinase inhibitors | Dabrafenib | Small molecule inhibitor | BRAF V600E mutations | Dabrafenib improves RR and PFS in lung cancer patients with BRAF V600E mutations [44] |
Vandetanib | Small molecule inhibitor | RET kinase activity | Vandetanib has been associated with significant antitumoral activity [46] | |
Checkpoint inhibitors | Nivolumab | Monoclonal antibody | PD1 | Nivolumab improves OS compared to docetaxel in the second-line setting in patients with squamous cell lung cancer [47] |
18.3 Targeted Therapies
18.3.1 Anti-EGFR
Activation of the EGFR pathway influences several oncogenic processes, including cell proliferation, resistance to apoptosis, migration, invasion, and angiogenesis. The EGFR protein is expressed in approximately 85 % of NSCLC, and its gene has become an interesting target for lung cancer therapy as a result of the development of the small tyrosine kinase inhibitors (TKIs) gefitinib, erlotinib, and afatinib. Gefitinib and erlotinib are reversible inhibitors that specifically target the EGFR protein, while afatinib is an irreversible inhibitor that binds covalently to EGFR and the other members of the ERBB family, including HER2, ERBB3, and ERBB4. Investigation has shown that the presence of a mutation in the exons coding for the tyrosine kinase domain provides with a protein that functions as a cancer driver that is sensitive to TKI activity. More than 40 different mutation sites have been identified in the EGFR gene, the most common being a deletion in exon 19 (Del19) and the point mutation in exon 21 (L585R); both of these account for more than 85 % of all detected mutations [27]. EGFR mutations can be found in approximately 17 % of Caucasian and 40 % of East-Asian patients with lung ADC and are more common in nonsmokers. PCR-based EGFR mutation testing is routinely performed in the diagnostic develop of non-squamous NSCLC [26]. The clinical activity of EGFR-TKI in patients harboring an EGFR mutation has been determined in a number of clinical trials in which more than 1800 patients with advanced EGFR mutation-positive lung cancer have been randomly assigned to receive either EGFR-TKI (erlotinib [28, 29], gefitinib [30–32] or afatinib [33]) or conventional platinum-based chemotherapy. All studies have presented considerable advantages for TKIs in terms of PFS compared with chemotherapy alone. The median PFS was 9.2–13.6 versus 4.6–6.9 months for TKIs compared to chemotherapy alone, respectively. Conclusively, in terms of OS, all trials have presented similar results for TKIs and chemotherapy alone, probably due to the large number of patient intersecting to TKIs after progression on chemotherapy. Nevertheless, it is notable that a combined analysis of the two trials comparing afatinib to chemotherapy has presented little but significant difference of 3 months in median OS favoring afatinib in the subgroup of patients (89 %) harboring the most common EGFR mutations, Del19 and L858R [34]. To improve the activity of TKIs in the first-line chemotherapy, combination strategies have also been investigated. In a recent study, erlotinib was managed alone or combined with the anti-vascular endothelial growth factor (VEGF) antibody bevacizumab as first-line chemotherapy in patients with EGFR-mutated lung ADC [35]. Median PFS was almost doubled by the addition of bevacizumab to erlotinib (16.0 vs. 9.7 months with erlotinib alone). OS data were not established at the time of publication. Further trials investigating the potential of this regimen are ongoing. Despite the high level of activity demonstrated by EGFR-TKI, patients’ tumor eventually progressed after a median period of nearly 10 months. Considerable research is currently focused on understanding the mechanism underlying the development of resistance to EGFR-TKI. The major mechanism is the acquirement of another mutation in exon 20 (T790M), which moderates the ATP-binding domain and significantly reduces the inhibition capabilities of TKIs. A T790M mutation is detected in more than 50 % of patients progressing on TKI. Other potential molecular changes with TKI resistance are MET amplification, HER2 amplification, PIK3CA mutations, and histological transformation into small-cell lung cancer.
Recently, third-generation EGFR inhibitors AZD9291, rociletinib, and HM61713 have produced extremely suggesting results. These drugs are specifically designed to target the T790M mutation but are also effective toward the other more common essential EGFR mutations. Expansion programs as well as randomized trials for this chemical substance are currently ongoing [36].
18.3.2 Anti-ALK
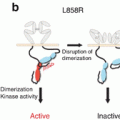
The discovery of ALK rearrangements represents another major breakthrough in the area of targeted therapies for NSCLC. Although this molecular change is detected in only 3–5 % of patients with lung ADC, considerable research has been dedicated to the clinical development of potent and specific ALK inhibitors, resulting in a time distance of only 4 years between the first account of ALK in lung cancer (in 2007) and approval by the Food and Drug Administration (FDA) of the first inhibitor, crizotinib (in 2011). The ALK gene is expressed as a result of fusion with another gene, the most common being EML4 in lung cancer. This fusion generates the expression of a kinase with high oncogenic potential that is mainly involved in cell proliferation (Fig. 18.1). The gold standard and FDA-approved method to detect ALK alterations in lung cancer is a break-apart fluorescence in situ hybridization (FISH) assay. However, FISH is relatively expensive and requires highly trained pathologists, since it may be difficult to identify and properly interpret the presence of split signals within the same chromosome, as often occurring with the ALK gene. Recently, an IHC assay targeting the effector protein has been developed and validated on a large scale and is likely to replace FISH as the standard diagnostic method for ALK expression in lung cancer [37]. In terms of clinical activity, crizotinib provides a better RR and PFS compared to chemotherapy in both the first-line (10.9 vs. 7.0 months with chemotherapy alone) and second-line setting (7.7 vs. 3.0 months with chemotherapy alone) [38, 39]. Again no differences in OS were observed between the crizotinib and chemotherapy arms, probably due to the frequent crossover to crizotinib at progression for patients assigned to receive chemotherapy.
As described with EGFR mutations, resistance to first-generation inhibitors in patients harboring ALK rearrangements is a matter of concern. The most common resistance mechanisms are the development of additional ALK mutations, ALK amplification, activation of EGFR signaling, and KIT amplification [40]. Furthermore, because penetration of crizotinib to the central nervous system (CNS) is poor, it is common to observe isolated CNS progression while other tumor localizations are still in reduction. Second-generation inhibitors, such as ceritinib, alectinib, and AP26113, have shown substantial activity against multiple ALK mutations and clinical effect in patients progressing on crizotinib. Thus far, ceritinib is the only approved ALK inhibitor besides crizotinib [41]. Several clinical trials comparing second-generation inhibitors to chemotherapy as well as adjacent association between the ALK inhibitors are ongoing to assess the best sequence of systemic therapy for ALK-positive lung cancer patients.
Approximately 1 % of lung ADC cases harbor a rearrangement in another fusion gene, ROS1. ALK and ROS1 share 70 % homology to crizotinib with similar response profiles [42]. It is very probable that the development of the other ALK inhibitors will lead to considerable benefit for the management of ROS1-positive lung cancer patients.
18.4 Other Targets
Using highly sensitive methods, BRAF mutations are detectable in up to 5 % of patients with lung ADC [43]. Treatment with specific inhibitors may be a valid option in this subgroup of patients, as confirmed by early experience of dabrafenib in lung cancer patients with BRAF V600E mutation (32 % RR and PFS of 5.5 months) [44].
RET rearrangements have also been described in lung cancer [45]. These are very infrequent genetic alterations (<2 %), but interesting nevertheless due to the availability of the specific inhibitor vandetanib, which has been associated with significant antitumor activity [46].
Finally, it is worth highlighting the recent breakthroughs that immunotherapy-based checkpoint inhibitors have had in lung cancer. Several monoclonal antibodies targeting the programmed cell death 1 (PD1) protein and its receptor PDL1 are currently under clinical development. Although no robust biomarkers have been identified yet as companion diagnostics for optimal patient selection, these drugs have shown a unique response and survival outcomes in lung cancer patients. Nivolumab, an anti-PD1 antibody, has been approved recently by the FDA for the treatment of SqCC. Approval was based on the preliminary results of a phase III trial in which nivolumab showed improved OS compared to docetaxel in the second-line setting [47].
18.5 Development of Anticancer Drugs and Strategies of the Success of Targeted Therapies
While targeted therapies have certainly improved survival rates among patients with cancer, the prognosis for those with metastatic disease remains extremely poor. This has focused interest on the best way to develop anticancer drugs while also maintaining a positive benefit risk ratio. One such approach is to ensure that tumor biopsies are obligatory, particularly in exploratory clinical studies [48]. This finding is closely linked to the multifaceted mutational processes operating in tumors [49, 50], whereby one small clone of a heterogeneous primary tumor may acquire metastatic abilities that are not seen in the surrounding tumor majority [51], thus potentially making translation IHC analysis insufficient.
Additional approaches ensure that patient baseline samples from clinical trials are carefully stored and catalogued, thus permitting initially unplanned retrospective analysis of newly characterized tumor biomarkers that may have appeared over the course of a long 4–5 year trial period and for cross validation purposes [48]. This would have the additional benefit of allowing subgroup analysis based on newly discovered and useful biomarkers/mutations and, as was the case with panitumumab and gefitinib, could result in approval of a drug that otherwise would have been declined.
Final approach to producing a successful targeted therapy is the addition of a translational research stage before the clinical trial starts. This is not as simple as taking samples and passing them to researchers, but must involve multidisciplinary team discussions to highlight firstly the potential mechanisms of primary or secondary drug resistance and secondly how these can be analyzed at a basic research aspect. Furthermore, this type of interaction can take the form of trying to produce predictive classifiers that can be used to identify patient subgroups that may not respond to treatment using some of the techniques. For example, a gene expression signature of responders versus nonresponders or DNA sequencing analysis to determine whether patients with specific gene mutations do not respond to the treatment can be performed.
Taken together, these fundamental ideas highlight the importance of patient selection and treatment-predictive biomarkers in confirming the success of targeted therapies [52] and signal the new age of personalized therapy. As part of this, clinical studies may in future include patients based on common molecular signatures and mutational patterns or functional properties before subgrouping based on histopathological diagnosis.
18.6 Conclusions
In this chapter, we have highlighted firstly how molecular markers can be examined and secondly how the data can be applied in clinical studies and routine clinical management of patients with NSCLC. Molecular markers are highly relevant in the adjuvant setting for NSCLC. These markers are also used to guide treatment in advanced lung cancer patients. Targeted therapies are not currently recommended in the adjuvant setting for lung cancers; however, a number of drugs are used in metastatic cases. Testing of the mutational status of the KRAS gene is recommended before commencing treatment with anti-EGFR therapies, as patients with mutations in this gene will not respond to therapy. The angiogenic inhibitor bevacizumab has also been approved for use in patients. However, the lack of a formal predictive biomarker for the drug means that it is not possible to select patients who are more likely to respond to treatment. Bevacizumab does not improve OS for several malignancies when combined with the best chemotherapy mainstays, and its added value is therefore questionable. EGFR and ALK inhibitors are commonly used to treat patients with NSCLC, meaning that diagnostic tests (such as qPCR and FISH/immunohistochemistry) to determine the presence of mutations and genetic deviations in these genes have become clinically useful before starting targeted treatment.
Generally, this evidence strongly suggests that in the immediate future, clinical diagnostics will use IHC and RNA and DNA-based methods to select patients who will benefit most from targeted treatment regimens.
Conflict of Interest Statement
No conflict of interest to declare.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree