Gastrointestinal stromal tumors (GIST) are the most common mesenchymal tumors of the gastrointestinal tract. Before the advent of tyrosine kinase inhibitors (TKIs) there were few treatment options available to patients with metastatic GIST. Surgery was the mainstay of treatment and the prognosis was dismal. With the advent of imatinib and second-line TKIs the prognosis of metastatic GIST has improved dramatically; however, there is still a need for therapies for patients with disease refractory to TKI therapy. Newer agents are under investigation and may have promise. This article discusses the current standard of care in terms of standard and investigational pharmacotherapy in the management of metastatic GIST.
Key points
- •
GIST represents a family of tumors with similar histologic features but different molecular drivers.
- •
The routine use of TKI therapies for the management of advanced GIST has led to increased survival for many patients.
- •
There are three approved agents for the treatment of GIST, and several other agents in which data support their use.
- •
Tumors that are refractory to TKIs remain a challenge and novel combination therapies being tested in the phase I setting may lead to new therapeutic options.
Tyrosine kinase inhibitors
Activating mutations in the KIT and platelet-derived growth factor-α (PDGFRA) genes have been implicated in the pathogenesis of gastrointestinal stromal tumors (GIST) ( Box 1 ). Most GIST have an activating mutation in the KIT gene commonly in exon 11 followed by exons 9, 13, and 17, which constitutively activates the gene product, a cell surface protein kinase receptor. Activating mutations in the PDGFRA gene occur in one-third of GIST tumors that lack KIT mutations. Tyrosine kinase inhibitors (TKIs) target mutated KIT and PDGFRA. Currently, imatinib is the agent of choice in the first-line setting followed by sunitinib in patients who are imatinib intolerant or resistant. Reports of GIST with alternate kinase mutations in B-RAF and RAS have been described. GIST without kinase mutations, classically called wild-type, represents a family of tumors associated with loss of succinate dehydrogenase (SDH) B protein expression with or without mutations in the SDH family of genes, occur in patients with neurofibromatosis, or may have a novel mechanism not yet identified. The mechanism of action of currently approved and investigational TKIs is summarized in Table 1 .
Tyrosine Kinase Inhibitors
Imatinib a
Sunitinib a
Regorafenib a
Nilotinib a
Sorafenib a
Masitinib
Vatalanib
Dovitinib
Pazopanib a
Cedarinib
Crenolanib
Hsp90 Inhibitors
BIIB021
AT13387
AUY922
Ganestespib (STA-9090)
PI3K-AKT-mTOR Inhibitors
Perifosine
Everolimus a
Sirolimus a
Temsirolimus a
Monoclonal Antibodies
Olaratumab
Insulin-like Growth Factor 1 Receptor Inhibitors
OSI-906 (Linsitinib)
a Commercially available.
| KIT | PDGFR α, β | VEGFR 1, 2, 3 | RET | FGFR 1, 3 | |
|---|---|---|---|---|---|
| Imatinib | + | + | |||
| Sunitinib | + | + | + | ||
| Regorafenib | + | + | + | + | + |
| Nilotinib | + | + | |||
| Sorafenib | + | + | + | ||
| Masitinib | + | + | |||
| Vatalanib | + | + | + | ||
| Dovitinib | + | + | + | + | |
| Papzopanib | + | + | + | + | |
| Cedarinib | + | ||||
| Crenolanib | + |
Tyrosine kinase inhibitors
Activating mutations in the KIT and platelet-derived growth factor-α (PDGFRA) genes have been implicated in the pathogenesis of gastrointestinal stromal tumors (GIST) ( Box 1 ). Most GIST have an activating mutation in the KIT gene commonly in exon 11 followed by exons 9, 13, and 17, which constitutively activates the gene product, a cell surface protein kinase receptor. Activating mutations in the PDGFRA gene occur in one-third of GIST tumors that lack KIT mutations. Tyrosine kinase inhibitors (TKIs) target mutated KIT and PDGFRA. Currently, imatinib is the agent of choice in the first-line setting followed by sunitinib in patients who are imatinib intolerant or resistant. Reports of GIST with alternate kinase mutations in B-RAF and RAS have been described. GIST without kinase mutations, classically called wild-type, represents a family of tumors associated with loss of succinate dehydrogenase (SDH) B protein expression with or without mutations in the SDH family of genes, occur in patients with neurofibromatosis, or may have a novel mechanism not yet identified. The mechanism of action of currently approved and investigational TKIs is summarized in Table 1 .
Tyrosine Kinase Inhibitors
Imatinib a
Sunitinib a
Regorafenib a
Nilotinib a
Sorafenib a
Masitinib
Vatalanib
Dovitinib
Pazopanib a
Cedarinib
Crenolanib
Hsp90 Inhibitors
BIIB021
AT13387
AUY922
Ganestespib (STA-9090)
PI3K-AKT-mTOR Inhibitors
Perifosine
Everolimus a
Sirolimus a
Temsirolimus a
Monoclonal Antibodies
Olaratumab
Insulin-like Growth Factor 1 Receptor Inhibitors
OSI-906 (Linsitinib)
a Commercially available.
| KIT | PDGFR α, β | VEGFR 1, 2, 3 | RET | FGFR 1, 3 | |
|---|---|---|---|---|---|
| Imatinib | + | + | |||
| Sunitinib | + | + | + | ||
| Regorafenib | + | + | + | + | + |
| Nilotinib | + | + | |||
| Sorafenib | + | + | + | ||
| Masitinib | + | + | |||
| Vatalanib | + | + | + | ||
| Dovitinib | + | + | + | + | |
| Papzopanib | + | + | + | + | |
| Cedarinib | + | ||||
| Crenolanib | + |
Food and Drug Administration–approved agents for GIST
Imatinib
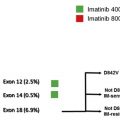
Imatinib (Gleevec) is a small-molecule TKI that exhibits inhibitory activity against ABL kinase, and KIT and PDGFRA receptor. Imatinib was first tested in a proof of principle trial in a patient with KIT exon 11 mutated metastatic GIST; the patient experienced a dramatic and durable response to therapy. Subsequent phase I-III studies demonstrated significant objective responses summarized in Table 2 . Phase I studies determined the maximum tolerated dose for imatinib to be 800 mg daily, with edema, including periorbital edema, diarrhea, nausea, vomiting, and myelosuppression being the main adverse events. The studies evaluated a range of doses: 400 mg daily, 600 mg daily, and 400 mg twice daily, with all studies demonstrating a high rate of objective responses. Two phase III studies compared 400 mg daily with 400 mg twice daily in advanced disease with similar overall response rate (ORR), complete response rate (CR), partial response rate (PR), and stable disease (SD) rates between the two arms. Patients were allowed to cross over from the 400-mg daily dose to 400-mg twice daily for progression; in one study 3% of patients who crossed over to the high-dose imatinib arm at the time of progression achieved a partial response and 28% achieved disease stabilization, albeit for a median progression-free survival (PFS) of 5 months. A meta-analysis of the phase III studies evaluated dosage of imatinib and showed that there was a PFS advantage for patients treated at the higher dose with a hazard ratio (HR) of 0.89 (95% confidence interval [CI], 0.79–1.00; P = .04); however, there was no difference seen in overall survival (OS) between the two arms. On subset analysis patients with KIT exon 9 mutations had a better ORR (47% vs 21%; P = .0037) and a significantly better PFS with an adjusted HR of 0.58 (95% CI, 0.38–0.91), again without a difference in OS between the two dose levels. The PFS benefit seen at the higher dose level in the meta-analysis was attributed to the benefit in patients with exon 9 tumors. Based on these data the current standard of care for advanced GIST is initiation of imatinib at a dose of 400 mg daily for all patients except for those with an exon 9 mutation and those who progressed on the lower dose of imatinib; these patients do better at a dose of 800 mg daily.
| Study | Study Type | Dosages Studied | Number of Patients | Results |
|---|---|---|---|---|
| van Oosterom et al, 2001 | Phase I | 400 mg qd, 300 mg bid, 400 mg bid, 500 mg bid | 40 | 54% PR 37% SD |
| Demetri et al, 2002 | Phase II | 400 mg qd vs 600 mg qd | 147 | No statistically significant differences in toxicity or response between the two doses Response rate in the 400-mg qd arm 49.3% PR, 31.5% SD, and 16.4% PD Response rate in the 600-mg qd arm 58.1% PR, 24.3% SD, and 10.8% PD |
| Verweij et al, 2003 | Phase II | 400 mg bid | 27 | 4% CR, 67% PR, 19% SD, 11% PD 73% free from disease at 1 y |
| Verweij et al, 2004 | Phase III | 400 mg qd vs 400 mg bid | 946 | Response rate in the 400-mg qd arm 4% CR, 45% PR, 32% SD, 13% PD Response rate in the 400-mg bid arm 6% CR, 48% PR, 32% SD, 9% PD After a median follow-up of 760 d, 263 (56%) of 473 patients in the once-a-day arm had progressed compared with 235 (50%) of 473 in the twice-a-day arm (HR, 0.82; 95% CI, 0.69–0.98; P = .026) OS was 85% at 1 y and 69% at 2 y in patients treated once a day, and 86% at 1 y and 74% at 2 y in those treated twice a day Increased dose reductions (77 [16%] vs 282 [60%]) and interruptions (189 [40%] vs 302 [64%]) in the twice-a-day arm |
| Blanke et al, 2008 | Phase III | 400 mg qd vs 400 mg bid | 694 | Response rate in the 400-mg qd arm 5% CR, 40% PR, 25% SD, 12% PD Response rate in the 400-mg bid arm 3% CR, 42% PR, 22% SD, 10% PD Median PFS was 18 and 20 mo in the once-daily and twice-daily arms, respectively Median OS was 55 and 51 mo in the once-daily and twice-daily arms, respectively No statistically significant differences in objective response rates, PFS, or OS |
Approximately 14% of GIST tumors have primary resistance to imatinib, progressing within 6 months of initiating therapy. These tumors most commonly are those with mutations in PDGFRA in exon 18, D842V, or those lacking mutations in either KIT or PDGFRA. Secondary resistance occurs in patients on long-term imatinib, greater than 6 months. Most of this resistance occurs because of clonal evolution. These clones express the primary mutation along with additional mutations that render them resistant to imatinib, leading to treatment failure and relapse; the secondary mutations occur within the same gene. The most common secondary mutations seen are in exons 13, 14, and 17 of the KIT gene and the D842V mutation in exon 18 of PDGFRA. The median time to progression on imatinib is approximately 2 years; however, some patients remain free of progression for greater than 10 years. Factors associated with long-term disease stability are good performance status and low base line neutrophil count. Factors that are associated with better OS include younger age, female gender, low neutrophil count, normal albumin, and good performance status. In addition, smaller tumor volume has also been associated with longer PFS.
Sunitinib
Sunitinib (Sutent) is a small-molecule TKI that inhibits vascular endothelial growth factor receptor (VEGFR) 1, 2, and 3; PDGFRA; PDGFR-β (PDGFRB); KIT; Flt3; RET; and CSF1R. In an early phase I study that looked at the use of sunitinib in patients with advanced malignancies, 6 of 22 evaluable patients had an objective response including one patient with imatinib-resistant GIST. A subsequent phase I-II study tested sunitinib in 97 patients who were intolerant to or had progressed on imatinib. A clinical benefit rate (CBR) defined as PR or SD lasting for more than 6 months was seen in 58%, 34%, and 56% of KIT exon 9 and 11, and KIT-PDGFRA wild-type mutations, respectively. The chances of achieving a PR with sunitinib was greater in tumors with an exon 9 KIT mutation compared with exon 11 KIT mutations (37% vs 5%). The median PFS was better for patients with exon 9 mutant and KIT-PDGFRA wild-type tumors compared with those with exon 11 mutations (19.4, 19, and 5.1 months, respectively). Median OS was also longer for patients with exon 9 mutants and KIT-PDGFRA wild-type tumors compared with the exon 11 mutants (26.9, 30.5, and 12.3 months, respectively) indicating that imatinib-insensitive mutations were more responsive to sunitinib in the second-line setting. Although those with KIT exon 11 mutations had less benefit, these patients represent a different population than those with imatinib-naive disease; rather, they represented those patients with clonal evolution and imatinib-insensitive secondary mutations. When secondary mutations were considered, the PFS for mutant KIT exon 11 patients with secondary KIT exon 13 or 14 mutation was 7.8 months with a median OS of 13 months and a CBR of 61% compared with a PFS of 2.3 months and a median OS of 4 months with a CBR of 15% for those with a KIT exon 17 or 18 mutation.
The pivotal phase III trial of sunitinib was a double-blind placebo-controlled study that enrolled patients with advanced GIST that had failed therapy with imatinib or who were intolerant to imatinib. Patients were treated with sunitinib at a dose of 50 mg daily for 4 weeks followed by a 2-week break in 6-week cycles. The primary end point of the study was time to tumor progression; secondary endpoints included PFS, OS, confirmed ORR, time to tumor response, duration of response, and duration of performance status maintenance. The results were impressive with the time to tumor progression for the sunitinib arm being 27.3 weeks, versus 6.4 weeks in the placebo arm with an HR of 0.33 (95% CI, 0.23–0.47; P <.0001). The study was unblinded at the first interim analysis and all patients on placebo were allowed to cross over to sunitinib. Even with crossover there was an OS advantage in the sunitinib arm with an HR of 0.49 (95% CI, 0.29–0.83; P = .007). The main toxicities were hematologic including anemia, thrombocytopenia and leucopenia, fatigue, diarrhea, nausea, vomiting, anorexia, stomatitis, and hand-foot syndrome.
The phase III trial of sunitinib administered sunitinib for 4 weeks followed by 2 weeks off drug. A schedule with intermittent breaks is less flexible and convenient for patients than a continuous dosing schedule. In addition, some patients experienced symptomatic progression or metabolic progression by fluorodeoxyglucose positron emission tomography scans during the time off drug. A phase II single arm study looked at the feasibility of daily dosing of sunitinib at a dose of 37.5 mg daily given continuously. The study demonstrated a CBR of 53%, including 13% PR and 40% SD. The median PFS was 34 weeks and the median OS was 107 weeks. The toxicity profile was similar to the phase III study. Based on these studies sunitinib is now the standard of care in patients with GIST who have failed imatinib, and is commonly given in a daily fashion.
Regorafenib
Regorafenib (Stivarga, BAY 73-4506) is a small-molecule TKI that inhibits VEGFR 1, 2, and 3; PDGFRB; FGFR1; KIT; RET; and BRAF among others. In a phase II trial of regorafenib, 33 patients with GIST tumors resistant to imatinib and sunitinib, but sorafenib-naive, showed a CBR of 75% (4 PR + 22 SD); the median PFS was 10 months and the median OS was not achieved at the time of reporting. Patients with a primary exon 11 mutation had a better PFS than patients with exon 9 mutations; however, there was no difference when they were compared with patients with wild-type GIST. The major toxicities that were reported were hypertension, hand-foot syndrome, hypophosphatemia, rash, fatigue, and diarrhea. In the recently reported phase III randomized controlled double-blind placebo-controlled GRID trial of regorafenib in patients with GIST tumors resistant to imatinib and sunitinib, the median PFS of patients on the regorafenib arm was 4.8 months and 0.9 months for those on placebo with an HR of 0.27 (95% CI, 0.18–0.39; P <.0001). Both these studies show promising results and regorafenib was approved in February 2013 as the third-line drug of choice.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree