Gastrointestinal stromal tumor (GIST) represents the most common mesechymal tumor of the gastrointestinal tract. Discovery of the relationship between unregulated KIT kinase and GIST transformation has led to diagnostic and therapeutic targeting. Imatinib is the recommended first-line treatment of metastatic GIST. In addition, the combination of surgery and imatinib for primary GIST is indicated in the adjuvant setting of high-risk patients and there may be benefit for this combination in the neoadjuvant setting. The success of molecular targeted therapy in GIST represents an important and exciting advance in solid tumor oncology.
Key Points
- •
The discovery of KIT mutations as a driver of GIST malignant transformation has resulted in an effective therapy with Imatinib KIT Tyrosine Kinase inhibitor.
- •
The adjuvant use of Imatinib has now been shown to improve both progression free and overall survival for high risk GIST.
- •
Neoadjuvant Imatinib in high risk GIST can be useful for tumor downstaging and subsequent organ sparing and less morbid surgical resection.
Introduction
Gastrointestinal stromal tumor (GIST) has emerged as the most commonly reported mesenchymal tumor of the gastrointestinal (GI) tract. This is due partly to the distinct defining characteristics of this tumor recognized by pathologists as a singular entity as well as an increase in reporting due to the unprecedented role of GIST as a model for the development of solid tumor targeted therapy. Although historically a purely surgical disease, this sarcoma is now managed through multimodality treatment considerations that often include a combination of surgical and systemic schemes to enhance disease control. This article reviews the background, prognostic factors, and current treatment recommendations for this rare but increasingly referenced malignancy.
Incidence
GISTs are the most common and prevalent sarcomas of the GI tract, accounting for at least 5% of all sarcomas and 80% of all GI mesenchymal tumors. The diagnosis of GIST has increased 25-fold over the past 20 years partly due to a reclassification of most GI smooth muscle tumors as GISTs. Within the Surveillance, Epidemiology and End Results patient registry, age-adjusted incidence of GIST diagnosis increased from 0.028 per 100,000 in 1992 to 0.688 in 2002. This is confirmatory to other studies that report an incidence of 7 to 14 GIST cases per 1 million in the general population.
Pathobiology
The novel observation and seminal article by Hirota and colleagues provided the basis for the subsequent expansive discoveries regarding the therapeutic targeting of the KIT oncoprotein in GISTs. This group first reported the link between activating mutations in the C-KIT proto-oncogene and its tyrosine kinase protein and the occurrence of GISTs. The hypothesis was initially based on observations that the interstitial cells of Cajal (ICC) found in the GI myenteric plexus express the KIT protein. They deduced that GIST seemed to have similar staining and ulrastructural characteristics to the ICC and found that GISTs almost universally demonstrate KIT gain-of-function mutations, suggesting that these tumors are a malignant variant of an ICC developmental cell. Subsequent to this discovery, gain-of-function mutations were found in platelet-derived growth factor receptor, alpha polypeptide (PDGFRA) in KIT-negative GISTs. It has been reported that approximately 80% of GISTs have an activating mutation in the KIT proto-oncogene and 5% to 8% have a mutually exclusive activating mutation in PDGFRA. These data have provided for advancements in both the diagnosis and therapy for GIST. These tumors can present a biologic continuum representing a spectrum from essentially benign small incidentally found bowel associated nodules to large highly metastatic tumors. Their prognosis and predictable clinical activity depend on presentation of size, mitotic activity, and molecular features.
GIST—Clinical Presentation
GISTs are most commonly diagnosed in adults between 50 to 80 years of age with a male-to-female ratio of 1:1. The majority of patients with GIST are symptomatic, with the most likely symptoms of pain and bloating related to an abdominal space–occupying mass. GI bleeding and anemia are less common and related to tumor mucosal erosion, which in itself is considered a negative prognostic factor. Because GISTs are generally extrinsic and exophytic ( Fig. 1 ), a large tumor can lead to intra-abdominal rupture or bleeding, signaling a poor prognostic event associated with an increased risk of tumor peritoneal implantation. The most common locations for GISTs are gastric (60%–70%), small bowel (20%–25%), colorectal (5%), esophagus (5%), and, rarely, omental, retroperitoneal, or mesenteric. Approximately 20% of GISTs are asymptomatic, small, and discovered incidentally on imaging or endoscopy. Endoscopic ultrasound indicates a typical appearance of a submucosal noncystic homogeneous mass and has proved useful in the evaluation of these visualized submucosal abnormalities. Although surgical resection is the standard therapy for localized GIST, and this is true for those tumors 2 cm and larger, there is some controversy regarding small incidental and asymptomatic gastric GISTs. Current recommendations suggest that gastric GISTs under 2 cm with low risk for malignant potential can be managed by active surveillance. Several pathology-based studies evaluating micro-GISTs (0.2–10 mm) found that they are widespread within specific populations and likely most do not progress to overt malignant disease. A study in Japan identified unsuspected GISTs less than 5 mm in 35% of 100 consecutive gastrectomy specimens and a study from Germany found GISTs less than 10 mm in size in 22.5% of autopsies in those greater than 50 years old. Although there is evidence that micro-GISTs frequently display an activating mutation in the C-KIT oncogene, it seems that multiple genetic alterations are necessary after the transforming KIT mutation before the malignant phenotype is fully expressed.
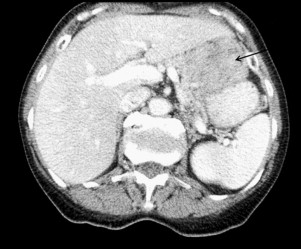
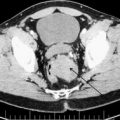
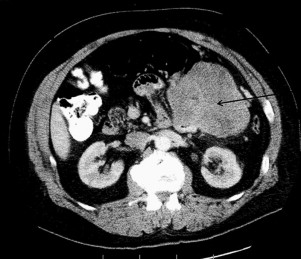
The clinical work-up of a suspected GIST should be similar to the evaluation of an undiagnosed intra-abdominal mass in any patient. Most are clinically apparent because of size and related symptoms of an intestinal associated mass with the occasional presentation of occult blood loss and anemia. Generally a cross-sectional imaging study is indicated to determine size, location, and associated organ involvement. A contrast-enhanced CT scan of the chest, abdomen, and pelvis is recommended to allow assessment of primary tumor extent and the possible presence of metastatic disease. A typical primary GIST images as an intestinal-based mass with the tumor bulk extrinsic to the bowel (unlike mucosal-based malignancies.) ( Fig. 2 ). In many instances, the primary tumor, although bulky, may be pedunculated, particularly if originating from the stomach, allowing for wedge resection rather than a formal gastrectomy. It is unnecessary to obtain a percutaneous image-guided biopsy of a suspected operable GIST because any potential tumor spillage from this procedure can increase the recurrence risk and the tumor can be pathologically characterized after surgical resection. Endoscopic biopsy of accessible tumors can provide for preoperative tissue diagnosis when clinically indicated.
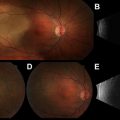
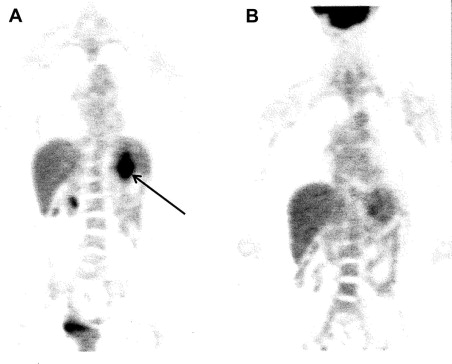
Functional imaging with fludeoxyglucose F 18 position emission tomography (PET) can be an important component to CT, because GISTs tend to be metabolically active and PET avid. Assessment of early metabolic changes, particularly in response to targeted KIT kinase inhibition, may be particularly helpful in a neoadjuvant treatment regimen ( Fig. 3 ) or as a diagnostic modality to detect recurrence or complement an ambiguous CT scan. In these specific instances, it is preferable to obtain a baseline PET scan as a comparison before initiation of specific therapy. Because of the added expense and varied interpretation of serial PET imaging, however, the CT evaluation of tumor density changes can often serve as a surrogate for PET evaluative measure of tumor response to kinase targeted therapy in either a primary GIST or in the metastatic setting.
Diagnosis
Elucidation of the pathology of GIST has been closely paralleled by therapeutic advancements. The specific diagnosis of GISTs is defined by morphology, ultrastructure, and immunohistochemistry. These tumors have common immunospecific lineage markers with ICC because both GISTs and ICC stain positive for KIT (CD117). Approximately 95% of GISTs stain for KIT; however, other immunomarkers can also be demonstrated, the most common of which is CD34. Some of the KIT-negative GISTs belong to a small subset oncologically driven by PDGFRA. In a clinically and morphologically suspected GIST that is KIT negative, a diagnosis can sometimes be confirmed by PDGFRA mutational analysis. These GISTs are more likely, however, to be clinically indolent, located within the stomach, and have epithelioid morphology.
The majority of GISTs are composed of a uniform population of spindle cells (70%), epitheloid cells (20%), or a mixed type (10%). GISTs generally have uniform cytologic features, because marked pleomorphism is uncommon, suggesting the expected biology cannot always be predicted by routine hematoxylin-eosin histology alone. KIT staining intensity is not related to either prognosis or response to therapeutic KIT specific inhibitors.
Molecular Classification
The molecular characterization of malignant solid tumors has become increasingly important for both diagnostic and therapeutic considerations. Along with the success of KIT kinase targeted therapy has come a considerable amount of data in GIST related to studies in genotype/phenotype relationships that have led to a molecular classification that is useful for treatment optimization. It is established that 70% to 80% of GISTs harbor a KIT gene mutation and that these mutations lead to constitutive activation of the transmembrane tyrosine kinase, which is an important therapeutic target. Approximately two-thirds of the mutations in KIT affect the juxtamembrane domain encoded by exon 11. These mutations disrupt the juxtamembrane scaffolding that normally prevents the kinase activation loop from alternating into an active configuration. These mutations can manifest as in-frame deletions, insertions, substitutions, or a combination. The deletions, in particular codon 557 or 558, are associated with a decrease in progression-free survival (PFS) and overall survival when compared with the other mutations in exon 11. Exon 11 mutated GISTs as a group, however, have the most favorable response as a drugable target for KIT kinase inhibition. The next most common KIT mutation (10%) occurs in the extracellular domain encoded by exon 9. These mutations recapitulate the structural change associated with the binding of the KIT natural ligand stem cell factor. This provides for a downstream signaling mechanism similar to that of a wild-type (lacking KIT or PDGFRA mutation) KIT and this has an effect on inhibitor sensitivity requiring dose escalation of imatinib mesylate, a first-line approved drug for KIT kinase inhibition. The GISTs harboring an exon 9 mutation are more commonly seen in the small and large intestines and are rarely seen in a gastric location. Mutations are uncommonly found (1%) in KIT exon 17 (activation loop) and in (1%) exon 13 (ATP binding site) ( Table 1 ).
| Genetic Type | Relative Frequency | Anatomic Distribution | Germline Examples |
|---|---|---|---|
| KIT mutation (relative frequency 75%–80%) | |||
| Exon 8 | Rare | Small bowel | One kindred |
| Exon 9 insertion AY502-503 | 10% | Small bowel and colon | None |
| Exon 11 (deletions, single nucleotide substitutions and insertions) | 67% | All sites | Several kindreds |
| Exon 13 K642E | 1% | All sites | Two kindreds |
| Exon 17 D820Y, N822K, and Y823D | 1% | All sites | Five kindreds |
| PDGFRA mutation (relative frequency 5%–8%) | |||
| Exon 12 (such as V561D) | 1% | All sites | Two kindreds |
| Exon 14 N659K | <1% | Stomach | None |
| Exon 18 D842V | 5% | Stomach, mesentery and omentum | None |
| Exon 18 (such as deletion of amino acids IMHD 842–846) | 1% | All sites | One kindred |
| KIT and PDGFRA wild type (relative frequency 12%–15%) | |||
| BRAF V600E | ∼7%–15% | ||
| SDHA, SDHB, SDHC, and SDHD mutations | ∼2% | Stomach and small bowel | Carney–Stratakis |
| HRAS and NRAS mutation | <1% | ||
| Sporadic pediatric GISTs | ∼1% | Stomach | Not heritable |
| GISTs as part of the Carney triad | ∼1% | Stomach | Not heritable |
| NF1 related | Rare | Small bowel | Numerous |
A minority of GISTs (8%) that lack a detectable gene mutation in KIT have a mutually exclusive activating mutation in PDGFRA, a transmembrane tyrosine kinase homolog of KIT. These mutations can occur within the juxtamembrane domain (exon 12), the ATP binding site (exon 14), or the activation loop (exon 18) and are characterized by a predilection for the stomach with variable and occasionally negative KIT expression. The most common PDGFRA mutation located in exon 18 (D842V) is resistant to present clinically available drugs for targeted tyrosine kinase inhibition (TKI).
Approximately 15% of GISTs do not display a definable KIT or PDGFRA mutation. These are classified by convention as wild-type GISTs. They represent a variety of different genomic changes but are not clinically distinct from mutant KIT or PDGFRA GISTs and generally have identical morphology, express KIT protein on IHC, and can occur anywhere in the GI tract. Although phosphorylated KIT is found in these GISTs, the mechanism of activation is unclear. BRAF, HRAS, and NRAS mutations have been identified as well as defects in succinate dehydrogenase. Approximately 50% of wild-type GISTs also show high expression levels of insulinlike growth factor.
Phase III clinical trials using imatinib and sunitinib (potent inhibitors of KIT and PDGFRA) have confirmed the relationship in metastatic GIST between tumor response and kinase genotype. The probabilities of initial exposure resistance to imatinib for KIT exon 11, KIT exon 9, and wild-type are 5%, 16%, and 23%, respectively. Of clinical relevance, the relationship between response and genotype indicates that the majority of GISTs (exon 11) are sensitive to standard-dose imatinib. Exon 9 mutations may require dose escalation of imatinib or a preference for sunitinib. Rare KIT mutations in the ATP binding domain are more sensitive to sunitinib whereas KIT activation loop mutations are generally resistant to either drug.
Recurrence Risk Factors
Based on histologic criteria alone, it is difficult to classify GISTs with reference to malignant potential and subsequent recurrence risk. The necessity of having a standardized classification for recurrence risk and metastatic potential is crucial to patient discussions regarding prognosis and potential for adjuvant therapy. Although surgical resection remains the cornerstone for the management of primary GIST, complete resection does not always lead to disease eradication. In an attempt to define the variables associated with surgical outcomes, several surgical, tumor, and pathologic related factors have been retrospectively analyzed for their correlation to recurrence ( Table 2 ). Completeness of surgical resection is a technical factor of prognostic importance as is tumor rupture. Patients with operable primary GIST who undergo R0 (complete gross and microscopic) resection have significantly longer survival than those who have incomplete resection. Tumor size, mitotic rate (as a surrogate for tumor grade), and tumor location have become the most significant variables for estimates of malignant potential and recurrence risk.
| Factors | Increased Risk a |
|---|---|
| Tumor size, mitotic rate, and tumor site | Gastric GIST |
| |
| |
| |
| |
| Small intestinal GIST | |
| |
| |
| |
| Duodenal GIST | |
| |
| |
| Rectal GIST | |
| |
| |
| Tumor size and mitotic rate |
|
| |
| |
| |
| KIT mutations |
|
| Location of primary GIST |
|
| Intraoperative factors |
|
| |
| Completeness of resection |
|
| |
| Tumor bulk |
|
a Approximate collective risk: very low—0%, low—3%–9%, moderate—12%–24%, high—55%–90%.
In 2001, a consensus conference attempted to correlate standards for recurrence risk based on tumor size and mitotic rate, which, by convention, was calculated as number of mitoses/50 high-power fields (HPF). An additional retrospective large database review reported tumor grade as a variable for risk stratification with low-grade GIST 5-year disease-free survival at 100% and high-grade disease-free survival at 15%. Tumor location has also been correlated to recurrence risk with small intestine and colorectal GISTs displaying the worst prognosis. The most comprehensive data collection was published in a review of more than 1900 patients from the Armed Forces Institute of Pathology where risk was evaluated according to tumor size, location, and mitotic rate, indicating a spectrum of what constitutes very low recurrence risk to high risk (see Table 2 ). Based on these data, nomograms have been developed to provide a more accurate individual patient risk assessment. GIST represents a continual spectrum of biologic potential from essentially benign tumors to aggressive malignancy. Even low-risk GISTs can result, however, in metastatic disease recurrence many years after initial resection and, therefore, patients need long-term and continued follow-up. It is recommended for those patients with resected intermediate-risk/high-risk primary GIST that an abdominal-pelvic imaging study be performed every 3 to 6 months for 3 years, then every 6 months until year 5, followed by yearly imaging. For low-risk patients, every 6 to 12 months for 5 years followed by yearly imaging is recommended. For very low risk GISTs, there is no recommended standard follow-up regimen.
Introduction
Gastrointestinal stromal tumor (GIST) has emerged as the most commonly reported mesenchymal tumor of the gastrointestinal (GI) tract. This is due partly to the distinct defining characteristics of this tumor recognized by pathologists as a singular entity as well as an increase in reporting due to the unprecedented role of GIST as a model for the development of solid tumor targeted therapy. Although historically a purely surgical disease, this sarcoma is now managed through multimodality treatment considerations that often include a combination of surgical and systemic schemes to enhance disease control. This article reviews the background, prognostic factors, and current treatment recommendations for this rare but increasingly referenced malignancy.
Incidence
GISTs are the most common and prevalent sarcomas of the GI tract, accounting for at least 5% of all sarcomas and 80% of all GI mesenchymal tumors. The diagnosis of GIST has increased 25-fold over the past 20 years partly due to a reclassification of most GI smooth muscle tumors as GISTs. Within the Surveillance, Epidemiology and End Results patient registry, age-adjusted incidence of GIST diagnosis increased from 0.028 per 100,000 in 1992 to 0.688 in 2002. This is confirmatory to other studies that report an incidence of 7 to 14 GIST cases per 1 million in the general population.
Pathobiology
The novel observation and seminal article by Hirota and colleagues provided the basis for the subsequent expansive discoveries regarding the therapeutic targeting of the KIT oncoprotein in GISTs. This group first reported the link between activating mutations in the C-KIT proto-oncogene and its tyrosine kinase protein and the occurrence of GISTs. The hypothesis was initially based on observations that the interstitial cells of Cajal (ICC) found in the GI myenteric plexus express the KIT protein. They deduced that GIST seemed to have similar staining and ulrastructural characteristics to the ICC and found that GISTs almost universally demonstrate KIT gain-of-function mutations, suggesting that these tumors are a malignant variant of an ICC developmental cell. Subsequent to this discovery, gain-of-function mutations were found in platelet-derived growth factor receptor, alpha polypeptide (PDGFRA) in KIT-negative GISTs. It has been reported that approximately 80% of GISTs have an activating mutation in the KIT proto-oncogene and 5% to 8% have a mutually exclusive activating mutation in PDGFRA. These data have provided for advancements in both the diagnosis and therapy for GIST. These tumors can present a biologic continuum representing a spectrum from essentially benign small incidentally found bowel associated nodules to large highly metastatic tumors. Their prognosis and predictable clinical activity depend on presentation of size, mitotic activity, and molecular features.
GIST—Clinical Presentation
GISTs are most commonly diagnosed in adults between 50 to 80 years of age with a male-to-female ratio of 1:1. The majority of patients with GIST are symptomatic, with the most likely symptoms of pain and bloating related to an abdominal space–occupying mass. GI bleeding and anemia are less common and related to tumor mucosal erosion, which in itself is considered a negative prognostic factor. Because GISTs are generally extrinsic and exophytic ( Fig. 1 ), a large tumor can lead to intra-abdominal rupture or bleeding, signaling a poor prognostic event associated with an increased risk of tumor peritoneal implantation. The most common locations for GISTs are gastric (60%–70%), small bowel (20%–25%), colorectal (5%), esophagus (5%), and, rarely, omental, retroperitoneal, or mesenteric. Approximately 20% of GISTs are asymptomatic, small, and discovered incidentally on imaging or endoscopy. Endoscopic ultrasound indicates a typical appearance of a submucosal noncystic homogeneous mass and has proved useful in the evaluation of these visualized submucosal abnormalities. Although surgical resection is the standard therapy for localized GIST, and this is true for those tumors 2 cm and larger, there is some controversy regarding small incidental and asymptomatic gastric GISTs. Current recommendations suggest that gastric GISTs under 2 cm with low risk for malignant potential can be managed by active surveillance. Several pathology-based studies evaluating micro-GISTs (0.2–10 mm) found that they are widespread within specific populations and likely most do not progress to overt malignant disease. A study in Japan identified unsuspected GISTs less than 5 mm in 35% of 100 consecutive gastrectomy specimens and a study from Germany found GISTs less than 10 mm in size in 22.5% of autopsies in those greater than 50 years old. Although there is evidence that micro-GISTs frequently display an activating mutation in the C-KIT oncogene, it seems that multiple genetic alterations are necessary after the transforming KIT mutation before the malignant phenotype is fully expressed.
The clinical work-up of a suspected GIST should be similar to the evaluation of an undiagnosed intra-abdominal mass in any patient. Most are clinically apparent because of size and related symptoms of an intestinal associated mass with the occasional presentation of occult blood loss and anemia. Generally a cross-sectional imaging study is indicated to determine size, location, and associated organ involvement. A contrast-enhanced CT scan of the chest, abdomen, and pelvis is recommended to allow assessment of primary tumor extent and the possible presence of metastatic disease. A typical primary GIST images as an intestinal-based mass with the tumor bulk extrinsic to the bowel (unlike mucosal-based malignancies.) ( Fig. 2 ). In many instances, the primary tumor, although bulky, may be pedunculated, particularly if originating from the stomach, allowing for wedge resection rather than a formal gastrectomy. It is unnecessary to obtain a percutaneous image-guided biopsy of a suspected operable GIST because any potential tumor spillage from this procedure can increase the recurrence risk and the tumor can be pathologically characterized after surgical resection. Endoscopic biopsy of accessible tumors can provide for preoperative tissue diagnosis when clinically indicated.
Functional imaging with fludeoxyglucose F 18 position emission tomography (PET) can be an important component to CT, because GISTs tend to be metabolically active and PET avid. Assessment of early metabolic changes, particularly in response to targeted KIT kinase inhibition, may be particularly helpful in a neoadjuvant treatment regimen ( Fig. 3 ) or as a diagnostic modality to detect recurrence or complement an ambiguous CT scan. In these specific instances, it is preferable to obtain a baseline PET scan as a comparison before initiation of specific therapy. Because of the added expense and varied interpretation of serial PET imaging, however, the CT evaluation of tumor density changes can often serve as a surrogate for PET evaluative measure of tumor response to kinase targeted therapy in either a primary GIST or in the metastatic setting.