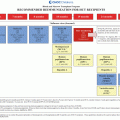
Cancer treatment risk factor
Potential late effect
Additional risk factors
Recommended evaluation
Evidence scorea
Mercaptopurine Thioguanine
Veno-occlusive disease (VOD)
Viral hepatitis
Screening
2A
Prior VOD
Siderosis
Mercaptopurine Thioguanine Methotrexate
Hepatic dysfunction
Viral hepatitis
ALT
Treatment before 1970
AST
Abdominal
Bilirubin
Radiation
Prior VOD
Abdominal radiation ≥ 30 Gy
Hepatic fibrosis Cirrhosis
Chronic hepatitis
Considerations for additional testing/intervention
Hematopoietic stem cell transplant (HSCT)
Hepatic dysfunction
Prior history of VOD
Prothrombin time
Chronic hepatitis
Higher radiation dose (≥40 Gy to atleast 1/3 of liver;
Screen for viral hepatitis
Cirrhosis
20–30 Gy to entire liver)
Ferritin/measure of liver iron burdenb
Iron overload
Alcohol use
Hepatitis A and B immunizations
Abdominal radiation
Cholelithiasis
Chronic GVHD
COG health links
1
Chronic hepatitis
1
Siderosis
2B
Steatosis
Multiple transfusions
Radiation to liver
Prior antimetabolite therapy
Alcohol use
Obesity
Pregnancy
Family history of cholelithiasis
Ileal conduit
Abdominal radiation
Total parenteral nutrition (TPN)
If a patient is a long term survivor at risk for transfusion related hepatitis, a screening Hepatitis B surface antigen (HBsAg), Hepatitis B core antibody (HBcAb) and/or Hepatitis C antibody are recommended [28]. If a patient is uncertain of their transfusion history, at a minimum Hepatitis C screening should be initiated, as the majority of long term survivors are lacking this basic screening and the outcome of the disease can be altered with medical and lifestyle interventions [54]. If a Hepatitis C antibody test returns as positive, further testing with Hepatitis C PCR is advised to confirm the initial findings [28].
10.3.4 Referral
Referral to a Gastroenterologist/Hepatologist is indicated for patients with persistent abnormal liver function tests or evidence of hepatomegaly either by physical exam or by imaging [28]. Survivors with confirmed Hepatitis C need close medical monitoring and should also have psychological assistance available, as patients with Hepatitis C report feelings of depression, stigma and anger associated with their disease [55]. This may be confounded by a history of childhood cancer, which is also associated with psychosocial distress [56].
10.3.5 Patient Education
All survivors, regardless of past exposures, should be educated on the potential for liver damage from medications, both over-the-counter and prescription, as well as from supplements [57, 58]. Acetaminophen is a well-established hepatotoxin, and patients should be educated that acetaminophen comes in various forms in combination over-the-counter pain and cold remedies [59]. Alcohol intake should be limited or eliminated completely as it is a major contributor to accelerating liver damage. Survivors should also be educated that emerging research is showing tattooing to be a risk factor for Hepatitis C, even among those with no other risk factors and having tattoos done in licensed parlors [60].
References
1.
Centers for Disease Control/National Center for Health Statistics (CDC/NCHS). (2010). National Hospital Ambulatory Medical Care Survey: 2010 Outpatient Department Summary Tables. http://www.cdc.gov/nchs/ahcd/web_tables.htm#2010
2.
Goldsby, R., Chen, Y., Raber, S., Li, L., Diefenbach, K., Shnorhavorian, M., et al. (2011). Survivors of childhood cancer have increased risk for gastrointestinal complications later in life. Gastroenterology, 140(5), 1464–1471. doi:10.1053/j.gastro.2011.01.049.PubMedCentralPubMedCrossRef
3.
Shadad, A., Sullivan, F., Martin, J., & Egan, L. (2013). Gastrointestinal radiation injury: Symptoms, risk factors and mechanisms. World Journal of Gastroenterology, 19(2), 185–198. doi:10.3748/wjg.v19.i2.185.PubMedCentralPubMedCrossRef
4.
Shafi, M., & Bresalier, R. (2010). The gastroinestinal complications of oncologic therapy. Gastroenterology Clinics of North America, 39, 629–647. doi:10.1016/j.gtc.2010.08.004.PubMedCrossRef
5.
Majhail, N. S., Rizzo, J. D., Lee, S. J., Aljurf, M., Atsuta, Y., Confim, C., et al. (2012). Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Bone Marrow Transplantation, 47(3), 337–341. doi:10.1038/bmt.2012.5.PubMedCentralPubMedCrossRef
6.
Nieder, M., McDonald, G., Kida, A., Hingorani, S., Armenian, S., Cooke, K., et al. (2011). National Cancer Institute–National Heart, Lung and Blood Institute/pediatric Blood and Marrow Transplant Consortium First International Consensus Conference on late effects after pediatric hematopoietic cell transplantation: Long-term organ damage and dysfunction. Biology of Blood and Marrow Transplantation, 17, 1573–1584. doi:10.1016/j.bbmt.2011.09.013.PubMedCentralPubMedCrossRef
7.
Molinaro, F., Kaselas, C., Lacreuse, I., Moog, R., & Becmeur, F. (2009). Postoperative intestinal after laparoscopic versus open surgery in the pediatric population: A 15 year review. European Journal of Pediatric Surgery, 19, 160–162. doi:10.1055/s-0029-1202858.PubMedCrossRef
8.
Lal, D., Foroutan, H., Su, W., Wolden, S., Boulad, F., & La Quaglia, M. (2006). The management of treatment-related esophageal complications in children and adolescents with cancer. Journal of Pediatric Surgery, 41, 495–499. doi:10.1016/j.jpedsurg.2005.11.065.PubMedCrossRef
9.
10.
Tuncer, H., Rana, N., Milani, C., Darko, A., & Al-Homsi, S. (2012). Gastrointestinal and hepatic complications of hematopoietic stem cell transplantation. World Journal of Gastroenterology, 18(16), 1851–1860. doi:10.3748/wjg.v18.i16.1851.PubMedCentralPubMedCrossRef
11.
Stinton, L., Myers, R., & Shaffer, E. (2010). Epidemiology of gallstones. Gastroenterology Clinics of North America, 39, 157–169. doi:10.1016/j.gtc.2010.02.003.PubMedCrossRef