Fig. 1
Histology of grade 1, well-differentiated, duodenal neuroendocrine tumor (gastrinoma) from a patient with MEN1-associated ZES. a Hematoxylin and eosin stain. b Immunohistochemistry for gastrin. c Chromogranin A. d Synaptophysin. MEN1 multiple endocrine neoplasia type 1, ZES Zollinger–Ellison syndrome
Once a diagnosis of ZES is suspected, the initial workup should include an evaluation of gastric acid hypersecretion and fasting serum gastrin levels . Because PPIs inhibit acid secretion, serum gastrin is commonly elevated as physiologic response to lowered intraluminal acidity. Therefore, PPIs and H2Rs should be held for 1 week and 2 days, respectively, if tolerated by the patient [2]. However, ZES patients can develop complications from acid hypersecretion and peptic ulcer disease during withdrawal from PPIs or H2-blockers; therefore, caution should be used when discontinuing these agents [13]. It is prudent to wait until peptic ulcer disease is significantly improved before holding PPIs or H2Rs for evaluation of serum gastrin. Patients with no active peptic ulcer disease can be treated with a high-dose H2-blocker while PPI is withdrawn for 3–7 days, then H2Rs should be stopped for 24 h to assess gastric acidity and fasting serum gastrin [14–17].
Criteria to diagnose ZES include (1) a fasting gastrin more than ten-fold higher than normal with a gastric pH less than 2 (40 % of patients) or (2) a fasting gastrin less than ten-fold, of upper limit of normal, gastric pH less than 2, and a positive secretin-stimulation test or gastric hypersecretion (60 % of patients). A positive secretin-provocative test (> 120 pg/ml increase) is frequently used to establish the diagnosis of ZES in questionable cases and has a sensitivity of 94 % and specificity of 100 % [18]. The later studies are needed in patients with less than a tenfold elevation of ZES, because a number of other diseases can also result in hyperchlorhydria/hypergastrinemia in this range, including Helicobacter pylori infections, renal failure, antral hyperplasia/hyperfunction, retained antrum syndrome, and extensive small bowel resections.
A negative secretin stimulation test is extremely rare in patients with ZES. If the secretin test is negative, but there is still a suspicion of ZES, either a repeated secretin stimulation test or a calcium stimulation test can be performed. The calcium stimulation test is used less commonly, as it is less sensitive (63 vs. 94 %) and has the potential for serious side effects from the intravenous infusion of calcium [18].
Because of the challenges in diagnosing patients with ZES and the potential complications from PPI withdrawal, the best approach may be to refer the patient to an institution with experience in ZES diagnosis [15, 16, 19].
Tumor Localization
The location of the gastrinoma and the extent of the disease are crucial for the management of ZES. Approximately, 80 % of gastrinomas are located within the “gastrinoma triangle,” which is defined by (1) the junction of the cystic and common bile ducts superiorly, (2) the junction of the second and third part of the duodenum inferiorly, and (3) the junction of neck and body of pancreas medially (Fig. 2 ) [20, 21]. Gastrinomas are frequently located in the duodenum (60 %), especially in patients with negative imaging studies. However, hepatic metastasis is more common when tumors are located to the left of the superior mesenteric artery, compared to those in the triangle [22].
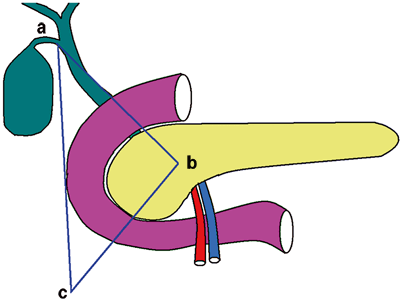

Fig. 2
Gastrinoma triangle. a The junction of cystic and common bile duct. b The junction of pancreatic neck and body. c The junction of second and third part of duodenum
Although thin-sectioned computed tomography (CT) and magnetic resonance imaging with contrast enhancement are the most commonly used preoperative localization studies because of widespread availability, the sensitivity of these modalities depends on the size of the lesions. Tumors less than 1 cm can be missed by cross-sectional imaging studies [16, 23]. Most authors recommend a biphasic or triphasic imaging protocol or pancreatic protocol [24]. Somatostatin receptor scintigraphy (SRS) in the USA is commonly performed using 111 indium-labeled somatostatin analogues with single-photon emission computed tomography (SPECT). Cross-sectional imaging studies detect 30–50 % of small primary gastrinomas (less than 1–2 cm) and 60–70 % of liver metastasis, whereas SRS with 111 indium-labeled somatostatin analogues has slightly higher sensitivity, at 60–70 % and 85–95 %, respectively [25–27]. The sensitivity of SRS using 111 indium is dependent on tumor size; 96 % of tumors larger than 2 cm could be detected, but, in one study, SRS missed a third of gastrinomas identified at surgery [28]. Recently, 68 gallium-labeled somatostatin analogues with positron emission tomography have been shown to be more sensitive than traditional 111 indium-labeled somatostatin analogues in detecting small NETs, including gastrinoma (Fig. 3 ) [29, 30]. Thus, this technique is likely to gain wide acceptance as the imaging procedure of choice for identifying NETs. This imaging is currently available in several centers in Europe and a few centers in the USA, including the NIH.
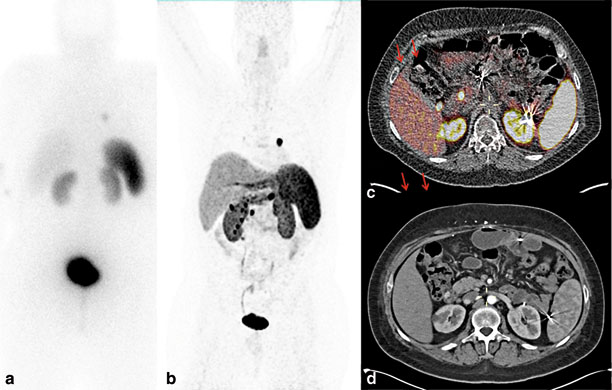

Fig. 3
Preoperative imaging studies in a patient with MEN1-associated ZES and metastatic neuroendocrine tumor. a 111 In somatostatin analogue scintigraphy demonstrated an avid left lung nodule with no other sites of disease. b 68-Ga DOTATATE scintigraphy demonstrates multiple abdominal-avid nodules, in addition to left lung nodule. c 68-Ga DOTATATE positron emission test/computed tomography revealed multiple, avid, paraduodenal lymph nodes and duodenal lesions. d Corresponding arterial phase of contrast-enhanced computed tomography. MEN1 multiple endocrine neoplasia type 1, ZES Zollinger–Ellison syndrome
Endoscopic ultrasound (EUS) has a high sensitivity for detection of pancreatic gastrinoma, ranging from 75 to 93 %, and enlarged peripancreatic lymph nodes [31]. However, the sensitivity of EUS for detection of duodenal gastrinomas is lower, ranging from 11 to 50 % [31]. The procedure allows cytologic evaluation of primary tumors as well as lymph nodes. Selective arterial secretagogue injection (SASI) test (with either secretin or calcium) and portal venous sampling was first described by Imamura et al. in 1987 to localize gastrinoma [32]. This test regionalizes the location of gastrinomas by selectively injecting secretagogue into arterial branches supplying the pancreas and duodenum and measuring the gastrin level by collecting venous samples from portal veins. A blush of contrast from angiography can reveal the location of NET as it is often very well vascularized. A step up in gastrin level helps localize the gastrinoma within the area supplied by the corresponding artery [33]. Although SASI has high sensitivity in localizing sporadic gastrinomas (86 %) and MEN1-associated gastrinomas (81 %) [34], it is used infrequently at present as other less invasive tests have improved diagnostic accuracy, and intraoperative maneuvers, such as intraoperative ultrasound, intraluminal illumination, and bimanual palpation, can successfully identify localized gastrinomas that preoperative localizing studies miss [34].
Classification
Prior to recent standardized nomenclature, NETs comprised various entities based on organ of origin, mechanism of development, function, histologic pattern, and biological behavior. These entities include carcinoid tumor, pancreatic islet-cell tumor, NET, and carcinoma. Recently, a number of organizations such as the World Health Organization (WHO), the European Neuroendocrine Tumor Society (ENETS), and the American Joint Committee on Cancer (AJCC)/Union for international Cancer Control (UICC) have developed standardized guidelines to classify NETs [41–47]. While differences in parameters exist among the classification systems, there is considerable overlap in the essential information used in each system; proliferative index is a fundamental characteristic used in grading system. While in concept, tumor grade and level of differentiation are related, subtle differences exist [45]. Tumor grade refers to inherent biological aggressiveness, whereas differentiation refers to the extent to which tumor cells retain or recapitulate nonneoplastic cellular features. For example, well-differentiated neuroendocrine neoplasms (grade 1 or grade 2 NETs) retain morphology similar to nonneoplastic neuroendocrine cells, including the presence of nesting, trabecular or organoid pattern, and strong expression of neuroendocrine differentiation makers such as Chromogranin A and synaptophysin. Ki-67-positive cells in grade 1 and grade 2 NETs are usually less than 20 %. In contrast, poorly differentiated NETs have less resemblance and are less organized than normal neuroendocrine cells. Proliferative index (Ki-67) in poorly differentiated NETs is frequently greater than 20 %, while immunostaining for neuroendocrine markers are often not avid and may only have focal staining [48].
Proliferative rate is typically assessed by performing mitotic count or by calculating the percentage of tumor cells that stain positive for Ki-67 expression. In 2010, the WHO incorporated proliferative indices such as mitotic count and Ki-67 expression as criteria to classify and consolidated all NETs into three groups: (1) well-differentiated NET grade 1 (mitotic count per 10 HPF < 2, Ki-67 < 2 %), (2) well-differentiated NET grade 2 (mitotic count per 10 HPF = 2–20, Ki-67 = 3–20 %), and (3) poorly differentiated neuroendocrine carcinoma grade 3 of small and large cell type (mitotic count per 10HPF > 20, Ki-67 > 20 %; Table 1 ).
Table 1
Grading criteria for gastrointestinal and pancreatic neuroendocrine tumors by WHO and ENETS classifications
Tumor grade | Proliferative indices | |
|---|---|---|
Mitotic count (per 10 HPF) | Ki-67 activity (%) | |
Grade 1 (low grade) | < 2 | < 3 |
Grade 2 (intermediate grade) | 2–20 | 3–20 |
Grade 3 (high grade) | > 20 | > 20 |
To further improve prognostic stratification of WHO classification for NETs , ENETS includes site-specific tumor, node, metastasis (TNM)-classifications, in addition to a three-tiered tumor grading system based on mitotic count and Ki-67 activity for GI and PNETs (see below) [42, 43].
An informative pathological report that aids in risk stratification should include diagnosis, tumor size, extent of primary tumor invasion, including lymphovascular, perineural invasion, resection margins, presence of necrosis, lymph node or distant metastases, tumor grade and differentiation, immunohistochemical staining for NETs, and proliferative rate (Ki-67 or mitotic count), as well as TNM staging [48].
Molecular Biology of Gastrinoma
The exact origin of the cells that give rise to pancreatic gastrinomas remains controversial. Some suggest that pancreatic gastrinomas may originate from islet and/or ductal cells [49, 50] . Duodenal gastrinomas originate from duodenal G cells. In patients with MEN1 or Werner’s syndrome, an increase in duodenal G-cell proliferation was observed concomitantly with loss of heterozygosity at the MEN1 locus (11q13) [50, 51]. The most common inherited syndrome associated with gastrinoma is MEN1, an autosomal-dominant syndrome caused by inactivating mutations in MEN1 gene that result in an alteration of menin protein. Germ-line mutation of this tumor suppressor gene is identified in 70–90 % of affected members of MEN1 families [52]. Because MEN1 syndrome can be diagnosed clinically in patients with two or more clinical features of MEN1 (pituitary tumor, pancreatic and/or duodenal NET, hyperparathyroidism), negative germ-line mutation does not exclude the diagnosis of MEN1 syndrome as the affected individual may have large intronic mutations that are not recognized by polymerase chain reaction [53, 54]. The importance of MEN1 tumor suppressor gene is further highlighted by the observation that 33–44 % of sporadic GI-PNETs harbor somatic inactivating mutations of MEN1 [55–57], that result in a loss of menin protein expression or an expression of truncated menin protein .
Menin is a 610-amino acid protein that is typically located in the nucleus but is also detected in cytoplasm and around telomerases [58, 59]. Menin binds to double-stranded DNA in a sequence-independent manner via its C-terminal region. Menin normally regulates transcription by stabilizing or modifying histone proteins ultimately resulting in inhibition of cell division [60]. The loss of a direct DNA binding by menin results in a failure to repress cell proliferation and cell-cycle progression [61] Menin interacts with numerous proteins, such as transcription factors, DNA repair factors, and cytoskeletal proteins. The first menin-interacting protein partner identified was JunD; interaction of menin with JunD forms a growth-suppressor complex [62]. Deacetylation of histones is an essential mechanism of repression. Molecularly, MEN1 syndrome can be visualized by the role of menin in direct regulation of cyclin-dependent kinase (CDK) inhibitors p27 and p18 expression. Menin activates transcription by a mechanism involving recruitment of mixed-lineage leukemia (MLL) protein, a histone methyltransferase, to the p27 and p18 promoters and coding regions . Loss of menin function from inactivating mutations in MEN1 results in downregulation of p27 and p18 expression, and thereby cell-cycle dysregulation resulting in abnormal cell growth and tumorigenesis in neuroendocrine cells [62]. There are over 25 proteins that interact with menin; however, the significance of all of these interactions for the development of NETs is largely unclear [63]. Functions of menin and interactions with JunD and MLL are shown in Fig. 4 .
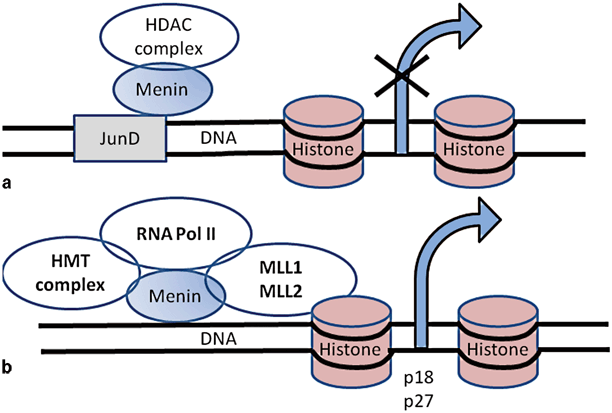

Fig. 4
The function of menin in transcription regulation by stabilizing or modifying histone proteins. a Menin interacts with JunD which binds to DNA and represses JunD-mediated transcription by recruiting histone-deacetylating protein complex. b Menin is a component of histone methyltransferase complex and interacts with MLL1 and MLL2. MLL and menin regulate expression of CDK inhibitors (p18 and p27). MLL mixed lineage leukemia, CDK cyclin-dependent kinase, HDAC histone deacetylase, HMT histone methyltransferase, RNA Pol II RNA polymerase II
Multiple genetic alterations have been described in sporadic NETs. In addition to MEN1 gene mutations, which commonly occur in sporadic NETs (up to 44 %), two other common mutations are death-domain associated protein ( DAXX; 25 %) and alpha thalassemia/mental retardation syndrome X-linked ( ATRX; 18 %) encode two subunits of a transcription and chromatin remodeling complex [55]. DAXX and ATRX proteins interact and both associate with histone H3 machinery to deposit the histones on the DNA. Both proteins are required for histone H3 incorporation at telomeres, a function that might be important for maintaining genome stability [55, 63] .
Clinically, mutations in MEN1 and DAXX/ATRX genes are associated with a better prognosis. Patients with mutations in both MEN1 and DAXX/ATRX survived at least 10 years, whereas patients lacking these mutations died within 5 years of diagnosis [55]. However, a recent study described the association of loss of DAXX or ATRX protein and alternative lengthening of telomeres with chromosomal instability, tumor stage, metastasis, reduced recurrent-free survival, and tumor-associated survival [64].
Approximately, 15 % of sporadic NETs have mutation in the genes involved in phosphoinositide 3-kinase/Akt/mammalian target of rapamycin (PI3K/Akt/mTOR) signaling pathway such as PTEN (7 %), TSC2 (9 %), and PI3KCA (1.4 %) [55]. Both Akt and mTOR play important role in multiple cellular processes including cellular proliferation, survival, and migration as well as glucose metabolism. Microarray expression profiling of PNETs showed downregulation of PTEN and TSC2, tumor suppressors involved in PI3K/Akt/mTOR signaling pathways, in the majority of primary PNETs [65]. In addition, lower expression was associated with shorter disease-free and overall survival [65]. These findings suggest that activation of PI3K/Akt/mTOR signaling pathway contributes to the development and behavior of NETs. Jiao et al. demonstrated that all PTEN-mutated tumors have secondary mutation of MEN1 and/or DAXX, suggesting that dysregulation of multiple pathways may be involved in the development of sporadic PNETs [55] .
Most GI-NETs and PNETs exhibit a high degree of vascularization. Pancreatic islet cells express high levels of proangiogenic proteins, such as vascular endothelial growth factor (VGEF), a factor that is critical for islet microvasculature and islet-cell function [66–68]. Other proteins including platelet-derived growth factor, c-kit, and those in the mTOR and hypoxic-induced factor 1 (HIF-1) pathways have a significant role in angiogenesis of NETs [69].
A unique feature specific to NETs is that low-grade benign-appearing PNETs exhibit higher microvascular density than high-grade tumors. In addition, unlike most epithelial tumors and carcinomas, higher microvascular density is associated with a survival benefit and better prognosis in PNETs [70]. However, high-grade PNETs still have activation of key regulatory pathways involved in hypoxic signaling and angiogenesis [69].
Treatment
Medical Control of Gastric Acid Hypersecretion
The most important initial treatment for a patient with ZES is to adequately control gastric hypersecretion with antisecretory drugs. PPIs, such as omeprazole, esomeprazole, rabeprazole, pantoprazole, and lansoprazole, are the medications of choice because of their potency and effectiveness as well as their long half-life that allows daily or twice-daily dosing [15, 23]. For treatment of ZES, the recommended dose for omeprazole, lansoprazole, and rabeprazole is 60 mg daily. The maximum dose varies based on response to treatment but can be as high as 240 mg per day in a divided dose. A divided dose is recommended once the required dosage to control the acid hypersecretion exceeds 120 mg per day. The recommended dose of pantoprazole and omeprazole is 40 mg twice daily with a maximum dose of 240 mg per day in a divided dose [2]. Intravenous PPIs are the treatment of choice in patients who cannot tolerate oral PPIs because of GI obstruction, ileus, vomiting, or postoperative restriction; PPIs, such as intravenous (IV) pantoprazole, can be administered twice daily, whereas a high-dose of IV H2Rs must be given continuously [71].
Long-term PPI treatment in patients with ZES is well tolerated with minimal side effects and has great compliance. The long-term complications include malabsorption of vitamin B12, iron, and calcium due to hypo or achlorhydria [71–73]. Patients who are on long-term PPI treatment should have their vitamin B12 checked periodically. Although not specific to patients with ZES, long-term use of PPIs is associated with increased risk of bone fractures [73, 74]. Unlike animal studies, prolonged exposure to hypergastrinemia in humans does not increase the incidence of gastric NETs (previously known as gastric carcinoids), as patients with sporadic ZES rarely develop gastric NETs [71, 75]. In contrast, gastric NETs develop in 23 % of patients with MEN1-associated ZES [76] and are not uncommon in chronic atrophic gastritis/pernicious anemia [77], suggesting that an accompanying defect may be needed in humans, such as loss of MEN1 or the presence of chronic atrophic gastritis, at least for the short-term development of gastric carcinoids (< 10 years).
Surgical Treatment for Localized Gastrinoma
Sporadic Gastrinoma
The goal of surgery is to provide biochemical cure (normalized fasting serum gastrin and negative secretin stimulation test) , prevent disease progression, and prolong survival. The impact of surgery depends on the stage of the tumor. Preoperative imaging studies localize primary gastrinoma in 60–70 % of patients. Surgical exploration should be offered to patients with sporadic gastrinoma even in those with negative preoperative localizing studies; however, primary gastrinoma may not be found in 15 % of these patients [2]. A recent study suggests that an experienced surgeon will find a gastrinoma in 98 % of patients, and 46 % will be cured in patients with negative preoperative imaging studies [78]. In this study, patients with negative imaging had a significantly longer delay from the onset of ZES to surgery compared with those with positive localizing studies (mean delay of 7.9 years), and 7 % of patients had liver metastases at the time of surgery [78]. In addition, patients with negative imaging studies had higher rates of duodenal gastrinoma and longer survival [78]. Surgical exploration should include (1) a wide Kocher maneuver to facilitate thorough examination of the head of the pancreas, uncinated process, and duodenum; (2) mobilization of the body and tail of the pancreas for bimanual palpation; (3) intraoperative ultrasound; (4) duodenal transillumination and duodenotomy for exploration of duodenal mucosa; and (5) removal of lymph nodes in the gastrinoma triangle.
Duodenotomy is indicated in all patients undergoing surgery for ZES and was first highlighted by N. W. Thompson. Duodenotomy is the most effective method of identifying duodenal gastrinomas, which represent 60 % of gastrinomas [79]. Biochemical cure increased from 30 to 60 % with duodenotomy and removal of duodenal gastrinoma [80, 81] . The choice of operation (enucleation versus anatomical resection) for pancreatic gastrinoma depends on the location of tumor and the proximity to pancreatic duct. Enucleation is the procedure of choice for superficial tumors or tumors that are not close to pancreatic duct. Systematic lymphadenectomy with retrieval of ≥ 10 lymph nodes during initial surgery may reduce the risk of persistent disease and improve survival [82] .
MEN1-Associated Gastrinoma
It has been well accepted that patients with MEN1-associated ZES who have primary hyperparathyroidism should undergo parathyroidectomy first to reduce gastrin stimulation from hypercalcemia [2]. On the other hand, the role of surgery in MEN1-associated gastrinoma continues to be controversial. Several studies have revealed that > 85 % of patients with MEN1-associated ZES have multiple duodenal gastrinomas that often are small (< 0.5 cm.) and are associated with lymph node metastasis in 40–60 % of patients [7, 34, 83]. Because biochemical cure in these patients is unlikely [34], and medical control of gastric acid hypersecretion is very effective, timing of surgical intervention and the extent of surgery are the subjects of ongoing debate because of the lack of prospective, controlled data. Some authors suggest that a cure can only be achieved by performing a pancreaticoduodenectomy; however, many of these studies lack long-term follow-up and inconsistent utilization of secretin stimulation testing [2, 7, 84]. Although pancreaticoduodenectomy provides a good chance for biochemical cure for gastrinoma in patients with MEN1 syndrome, recent guidelines by Thakker et al. do not recommend this procedure because of associated morbidities and because lesser operations in these patients are associated with excellent long-term survival [11]. Thompson et al. reported eugastrinemia in 68 % of patients with MEN1-associated ZES undergoing aggressive targeted resection, which included a distal pancreatectomy, enucleation of any head, uncinate, and duodenal tumors as well as peripancreatic lymphadenectomy (the Thompson procedure) [85]. Less extensive surgery that focuses on identified lesions (enucleation or segmental resection of involved pancreas, with peripancreatic and periduodenal lymphadenectomy) has been currently used by groups at the NIH and the Ohio State University [86]. The rationale supporting this approach includes the indolent course of ZES in patients with MEN1, and the observation that most patients have longevity without undergoing radical resection that can decrease quality of life because of short- and long-term complications. Furthermore, a survival benefit can be achieved when no residual tumor is grossly visualized without normalization of postoperative gastrin [86]. The role of surgery for patients with MEN1-associated gastrinoma is largely determined by preoperative imaging studies. Surgery should be considered if lymph node metastasis is suspected, even in the absence of distant metastasis. While previous reports from the NIH recommended surgery in patients with tumors > 2–2.5 cm [87, 88], others do not use size to determine the time of intervention and consider surgery if localizing studies identify suspicious lesion(s) [2].
Treatment of Advanced, Metastatic Gastrinoma
Despite indolent clinical course, most gastrinomas are malignant, and the disease recurrence or progression occurs in 40–60 % of patients with sporadic disease, while most MEN1-associated ZES will not be cured surgically [2, 23]. Because effective treatments are available to control symptoms from hormonal overproduction, the treatment strategy for patients with advanced or metastatic gastrinoma (and other NETs) should target tumor progression both locoregionally and systemically. Because of the rarity of ZES, most studies that have patients with advanced gastrinoma include all other different kinds of PNETs since the treatments for advanced PNETs are similar. The presence of liver metastasis is one of the most important prognostic factors in patients with ZES. The 10-year survival for patients with diffuse metastatic gastrinoma in the liver ranges from 15 to 35 % [86, 89, 90].
Fasting serum gastrin levels greater than 700 pg/ml are associated with poor survival; only 20 % of patients survive 10 years compared with 100 % of patients with a fasting gastrin levels less than 700 pg/ml [91]. Other factors associated with a poor survival for PNETs are extensive liver metastasis (> 70–75 % of liver volume involved), polyhormonal secretion (particularly adrenocorticotropic hormone; ACTH), extrahepatic metastases (in particular, bone metastases), and an unresected primary tumor.
There are several treatment modalities available for patients with advanced PNETs, including gastrinoma , such as cytoreductive surgery, liver-directed therapy (radiofrequency ablation (RFA), embolization, chemoembolization, or radioembolization), liver transplantation, biotherapy (somatostatin analogues, interferon alpha), targeted-molecular therapy (everolimus and sunitinib), and peptide receptor radionuclide therapy (PRRT) .
Cytoreductive Surgery
The benefit of cytoreductive surgery in patients with metastatic gastrinoma is difficult to assess because most series combine PNETs, including gastrinoma and carcinoid tumors. In addition, there have been no case–control studies, and selection bias in retrospective studies can be a contributing factor for survival benefit. It is important to confirm that surgical candidates have (1) well-differentiated NETs (G1 or G2), (2) no extra-abdominal metastasis, and (3) no diffuse or unresectable peritoneal carcinomatosis. It is generally accepted and recommended by ENETS that cytoreductive surgery can result in long-term survival in selected cases, and a survival benefit has been reported when at least 90 % of visible metastatic tumors are removed or ablated [2, 92–94]. Resection of the primary tumor, in the presence of unresectable liver metastasis, may be considered to avoid local complications such as intestinal obstruction, mesenteric retraction, and hemorrhage. If surgery is indicated, a cholecystectomy may be considered to prevent ischemic complications of the gallbladder following (chemo)embolization. Gallstones are less frequently observed with somatostatin analogue SSA therapy than previously expected, thus preventive cholecystectomy may not necessarily be required [92].
Liver-Directed Therapy
The indication for liver-directed therapy in patients with metastatic gastrinoma is usually progressive liver involvement, since ZES and gastric acid hypersecretion can be well managed medically. There are several modalities of liver-directed therapy for patients with advanced NETs. Percutaneous RFA or intraoperative RFA uses the thermal energy to ablate the tumor. Despite a high response rate of ablated tumor (80–95 %), it remains unclear if patient survival is comparable to medical therapy or palliative liver resection due to lack of randomized clinical trials. Because most hepatic metastasis from NETs have abundant blood supply from the hepatic artery, a selective hepatic artery embolization using a coil or beads coated with a chemotherapeutic agent or radioactive material is a good option for patient with bi-lobar liver metastasis and/or large tumors (> 5 cm), situations which are considered unsuitable for RFA. Post-embolization complications are not uncommon. These include tumor necrosis and abscess, gallbladder necrosis, pancreatitis, and hepatic transaminitis.
The choice of an ablative procedure (RFA, chemoembolization, radioembolization, etc.) depends on the experience of the operator, location(s), number, and size of the tumors. Recurrence and progression after RFA for metastatic NET are common (60 % in 10-month follow-up) [95].
Biotherapy
This includes somatostatin analogues and/or interferon. These agents have a tumoristatic effect and result in disease stabilization in 40–80 % of patients, while less than 15 % of tumor response rate [92, 96]. The clinical benefit of the antiproliferative effect of somatostatin analogues in patients with advanced gastrinoma or PNETs is unknown. A prospective, randomized, double-blind study in GI midgut carcinoid tumors (PROMID trial) using long-acting octreotide demonstrated a significantly longer time to tumor progression in the treatment group (14.3 vs. 6 months) [97]. The ENETS 2012 guidelines conclude that somatostatin analogues may provide some benefit in patients with advanced well-differentiated PNETs and that interferon should be considered in those patients if the PNET is somatostatin-receptor negative [92]. The Controlled Study of Lanreotide Antiproliferative Response in Neuroendocrine Tumors (CLARINET) trial has been designed to assess the benefit of somatostatin analogue in patients with advanced nonfunctioning GI-NETs and PNETs. Lanreotide was associated with significantly prolonged progression-free survival (median progression-free survival, not reached vs. 18.0 months, p< 0.001), compared to placebo. The estimated rates of progression-free survival at 24 months were 65 % in the lanreotide group and 33 % in the placebo group [98].
Molecular-Targeted Therapy
Everolimus and sunitinib are currently approved treatment options for progressive, locally advanced, or metastatic well-differentiated PNETs, including gastrinoma . Although tumor remission is rare, 60–80 % of patients experience disease stabilization [92]. The use of everolimus for PNETs has been approved by Food and Drug Administration (FDA) based on a large ( n = 410) prospective, randomized, placebo-controlled phase III trial that demonstrated significantly longer progression-free survival in the treatment group (11.0 vs. 4.6 months) [99]. However, no difference in overall survival was observed, likely because of the crossover. Everolimus has an acceptable safety profile and may be considered as the first-line therapy in select patients with advanced well-differentiated (low or intermediate grade) PNETs. The most frequent side effects included stomatitis (64 %), rash (49 %), and diarrhea (34 %), and the most frequent grade 3/4 side effects were stomatitis (7 %), anemia (6 %), and hyperglycemia (5 %) [99].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree