Gastrinomas are gastrin-secreting neuroendocrine tumors (NETs), usually located in the duodenum or pancreas, that result in hypergastrinemia. This leads to excessive gastric acid secretion and the resultant clinical manifestations, such as refractory PUD (with sequelae like bleeding, perforation, and obstruction), gastroesophageal reflux disease, and diarrhea.1,2 Gastrinomas are associated with multiple endocrine neoplasia type 1 (MEN-1) in about 25% of cases and occur sporadically the other three-quarters of the time.2,3
In 1955, Zollinger and Ellison published a report of two patients with refractory jejunal ulcers and concomitant pancreatic islet cell tumors. They proposed a “clinical entity consisting of hypersecretion, hyperacidity, and atypical peptic ulceration associated with non-insulin-producing islet cell tumors of the pancreas.”4 In fact, at least 16 reports of similar cases were published between 1908 and 1952 (six of which were referenced by Zollinger and Ellison), but Zollinger and Ellison were the first to suggest that an ulcerogenic factor was being produced by the islet cell tumor.5,6 They erroneously speculated that this factor was glucagon, and it was not until 5 years later that Gregory and Tracy extracted gastrin from the pancreatic tumor of a patient with Zollinger–Ellison syndrome (ZES).7
During the first decade after Zollinger and Ellison’s landmark publication, more than 250 cases were described and a tumor registry was established. In Ellison and Wilson’s 1964 review of these cases, they recognized that there was a frequent occurrence of multiple tumors or liver metastases, and they concluded that a total gastrectomy was the best surgical option as definitive ulcer management.5,8 At the same time, Oberhelman9 was the first to report a duodenal, rather than pancreatic, location for gastrinomas. He advocated local excision of the tumor (including pancreaticoduodenectomy if necessary) rather than total gastrectomy.10 By the mid-1970s, the tumor registry included more than 800 patients, and the majority of morbidity and mortality for these patients was related to PUD. It was understood that gastrinomas commonly occurred at multiple sites and were difficult to localize, and therefore removing the target organ by total gastrectomy remained the preferred operation.5,11
This philosophy changed with the advent of H2-receptor antagonists. Several reports in the mid-1970s described successful reduction of gastric acid secretion in patients with ZES who were treated with metiamide or cimetidine.12–16 In 1978, McCarthy declared cimetidine to be an acceptable alternative to total gastrectomy.17 Refractory PUD became even less common in the proton-pump inhibitor era.18
With this paradigm shift to medical management of gastric acid secretion, survival became more dependent on tumor progression and metastatic disease rather than PUD sequelae, and the surgical goal became curative resection of the gastrinoma.19 However, localization remained a challenge. Preoperative CT scan, ultrasound, and angiography were neither sensitive nor specific, and intraoperative exploration remained the best method to identify gastrinomas. In a prospective series by Norton et al in 1986,20 a tumor was found in only 62% of patients, even with a thorough exploration and the use of intraoperative ultrasound, and there was only a 30% cure rate at 6 months. In 1989, Thompson and colleagues21 described detection of tumors as small as 2 mm by eversion of the duodenal mucosa and digital palpation, thus establishing the importance of routine duodenotomy during exploration for gastrinoma. In the 1990s, preoperative localization was further improved with the introduction of somatostatin receptor scintigraphy (SRS).22–24
Nearly two decades later, despite improved understanding and technological advances, gastrinoma remains a complex and challenging condition to diagnose and manage.
The incidence of gastrinoma is 0.5 to 2 per million people per year.25,26 Though rare, gastrinomas are the most common type of functional, potentially malignant pancreatic endocrine tumor, accounting for about one-third of these.26
Gastrinoma is diagnosed between the ages of 20 and 60 in 90% of cases, with mean age at diagnosis being 41 to 54 years.2 Patients with MEN-1 tend to have a younger age of onset, with a median of 33.2 years.26 Gastrinomas are 1.3 to 1.5 times more common in males than females.3
Approximately 25% of patients with gastrinoma have MEN-1, and conversely gastrinoma occurs in approximately 50% of patients with MEN-1.25 In these patients, the gastrinomas are almost always multiple and 70% to 100% are located in the duodenum.25,26 All patients diagnosed with ZES should be screened for MEN-1 with a thorough family history and laboratory tests including calcium, parathyroid hormone, prolactin, and pancreatic peptide (especially with evidence of pancreatic tumors).25,26
Specific risk factors for sporadic gastrinoma are not well established. Based on a 47-year observation of 39 gastrinoma patients, Wilson and colleagues27 have proposed that alcohol abuse is a risk factor for sporadic duodenal wall gastrinomas. However, these data are limited by the inherent biases of observational studies, and further investigation is required to determine whether the demonstrated association between alcohol consumption and duodenal gastrinoma is indeed causative.
The MEN-1 syndrome is inherited in an autosomal dominant fashion by a germline mutation of the MEN-1 gene, which is a tumor suppressor gene located on the short arm of chromosome 11 (11q13).3,28 The MEN-1 gene encodes for the protein menin, which is a widely expressed 67-kDa protein that plays an important role in transcriptional regulation and cell growth. More than 1300 mutations in MEN-1 have been identified, most of which result in loss of function of menin, but there is not a clear genotype/phenotype correlation.
Mutations in the MEN-1 gene may play a role in the pathogenesis of sporadic gastrinomas, with somatic mutations reportedly found in 17% to 58% of cases. The majority of these involve deletions in the exon 2 region of the MEN-1 gene.3 In a 1997 NIH study of 28 sporadic gastrinomas, a somatic mutation of MEN-1 was detected in 33% and a loss of heterozygosity producing a single copy of the MEN-1 gene was found in 93% of these cases. These authors concluded that the absence of one allele coupled with an inactivating mutation of the second allele was an important mechanism of gastrinoma tumorogenesis.29 Interestingly, MEN-1 gene mutations in sporadic gastrinoma do not seem to predict biological behavior. Unlike in patients with the MEN-1 syndrome, the presence or absence of a MEN-1 mutation in sporadic cases has not been demonstrated to correlate with clinical presentation, tumor location, risk of metastatic disease, or postoperative recurrence.30,31
Many other genetic mechanisms have also been described in the development of sporadic gastrinomas. Hypermethylation of the p16 tumor suppressor gene on chromosome 9p21 can be found in up to 52% of sporadic gastrinomas. The p16 gene encodes for p16, which is a cyclin-dependent kinase inhibitor that arrests the cell cycle. Mutation of the p16 gene does not seem to correlate with clinical characteristics or tumor behavior.32 Other mutations, however, do appear to predict biologic behavior and propensity for metastasis. Loss of heterozygosity in chromosome 1q has been described in 44% of gastrinomas and is associated with aggressive growth (defined as >25% increase in tumor volume in 1 month), presence of hepatic metastases at presentation, and development of postoperative hepatic metastases after gastrinoma resection.33 X-chromosome loss of heterozygosity (identified in 56% of gastrinomas from female patients),34 increased expression of insulin-like growth factor 1,35 and overexpression of HER-2/neu36 have all been shown to have potential prognostic significance in sporadic gastrinomas by correlating with increased tumor growth and likelihood of metastatic spread.
It has also been suggested that duodenal and pancreatic gastrinomas have entirely separate genetic backgrounds. In a study including 15 patients with duodenal tumors and 11 patients with pancreatic tumors, Fendrich et al showed that 100% of the duodenal gastrinomas studied expressed Sonic hedgehog (Shh) and 0% expressed pancreatic-duodenal homeobox-1 (Pdx1). Conversely, 100% of pancreatic gastrinomas expressed Pdx1 and 0% expressed Shh.37 This genetic difference may be useful in analysis of resected metastases when the primary gastrinoma is not successfully localized.
Gastrin is a polypeptide hormone that is secreted by the neuroendocrine G-cells of the gastric antrum under normal physiologic circumstances. Gastric distension, the presence of amino acids within the stomach, and increased vagal activity all stimulate its release. It then acts on the gastric chief cells to stimulate gastric acid secretion. Increased acid content within the stomach normally produces negative feedback and inhibits the secretion of gastrin.2,3
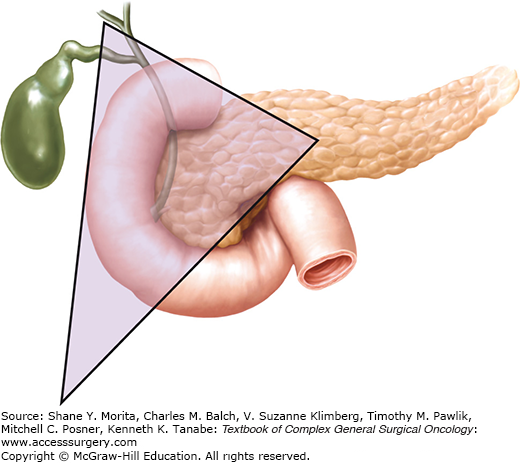
Gastrinomas occur either in the pancreas or the duodenum. In most of the early studies, gastrinomas were considered to be primarily pancreatic tumors. More recent series, however, suggest that 40% to 90% of all gastrinomas are located in the duodenum, and indeed duodenal gastrinomas may be three to ten times more common than pancreatic gastrinomas.25,38 Appreciation of this increased incidence is attributable to the standard inclusion of duodenotomy during an exploration for ZES, which did not become routine until the early 1990s.21,25 Duodenal gastrinomas tend to be small: 49% to 80% of tumors are less than 1 cm, with a mean size of 0.6 to 1.3 cm.3 They are most often found in the first portion of the duodenum, including the bulb, and they decrease in frequency from proximally to distally. Although it was previously believed that pancreatic gastrinomas were more likely to occur in the head or the tail of the pancreas, they seem to occur with equal distribution throughout the gland.26 Pancreatic gastrinomas tend to be larger, with a mean size of 2.7 to 3.2 cm.3 Regardless of whether they are duodenal or pancreatic, 70% to 90% of gastrinomas are found in the “gastrinoma triangle” of the right upper quadrant.25,26 This region, originally described by Stabile et al in 1984,39 is defined superiorly by the junction of the cystic duct and the common bile duct, medially by the junction of the pancreatic neck and body, and inferiorly by the junction of the second and third portions of the duodenum (Fig. 47-1).
FIGURE 47-1:
The gastrinoma triangle, originally described by Stabile et al in 1984, is defined superiorly by the junction of the cystic duct and the common bile duct, medially by the junction of the pancreatic neck and body, and inferiorly by the junction of the second and third portions of the duodenum.
Rarely, primary gastrinomas can be found in other intra-abdominal locations such as the stomach, jejunum, mesentery, omentum, biliary tree, liver, splenic hilum, renal capsule, ovary, and lymph nodes (although this is controversial); in <0.3% of cases, gastrinomas may arise in extra-abdominal locations, including the heart and lung.1
The pathogenesis, and even the cell of origin, of gastrinomas has not been fully elucidated.1 They have previously been categorized as pancreatic islet cell tumors, but they appear to arise from pluripotent neuroendocrine stem cells rather than islet cells.25 However, there is still dissent in the literature as to the precise mechanism of neoplastic development, with some authors demonstrating initiation of tumorogenesis in the ductal acinar system,40 and others proposing that gastrinomas arise from precursor G-cell hyperplasia.41
Histologically, gastrinomas tend to be well differentiated, with small, uniform cells arranged in a trabecular and pseudoglandular pattern.26 On H&E staining, they have fine, granular, eosinophilic cytoplasm and few mitoses.25 Immunohistochemistry is critical: staining for chromogranin A and synaptophysin confirms neuroendocrine origin, and a gastrin-specific stain helps establish the diagnosis of gastrinoma. Specimens should also be analyzed for proliferative index, by Ki67 staining, and mitotic index, by counting 10 HPF.26 The microscopic appearance of duodenal and pancreatic gastrinoma is generally indistinguishable, though there does seem to be a higher proliferation and angioinvasion rate in pancreatic gastrinomas.42 Unless a gross invasion of normal tissues is seen, histologic differentiation between benign and malignant gastrinomas usually cannot be made.25
The most common sites of gastrinoma metastasis are lymph nodes and the liver.25 Metastatic disease also occurs in the bones (often with concurrent liver metastases), and has been reported in the peritoneum, omentum, and lung.3 As many as two-thirds of gastrinoma patients have disease involvement of regional lymph nodes demonstrable at the time of initial exploration. A smaller, but still significant, number of patients (8% to 22%) have hepatic metastases at the time of initial presentation.3 Whereas pancreatic and duodenal gastrinomas have been shown to metastasize to regional lymph nodes at similar rates, pancreatic gastrinomas are more likely to metastasize to the liver.43
Prior to the widespread availability of antisecretory medications (histamine H2 antagonists and proton pump inhibitors [PPIs]), patients with ZES generally presented with severe consequences of PUD such as perforation, upper gastrointestinal hemorrhage, gastric outlet obstruction, or esophageal stricture. However, even in the era of PPI therapy, most patients with gastrinomas still present with symptoms of gastric acid hypersecretion, and 98% of patients are symptomatic at presentation.3 The most common initial symptoms are abdominal pain (47% to 75%), diarrhea (30% to 73%), nausea/vomiting (30% to 45%), and heartburn (41% to 56%). Gastrointestinal bleeding (hematemesis, melena, or both) is present in 6% to 27%, and 17% of patients experience weight loss.26,44,45 In general, patients tend to experience a constellation of symptoms, with more than three-quarters presenting with two to four of the above-mentioned complaints.44 However, in a review of 261 patients, 11% had one symptom only,44 and in 10% diarrhea was the sole presenting symptom.1,46 Tumor extent and tumor location do not appear to influence the type or severity of symptoms.44
The initial diagnosis of gastrinoma is rarely correct, and, even in the current era, it is delayed an average of 5 to 9 years after onset of symptoms.1,26,44 Many patients with ZES are treated empirically with PPIs early in the disease process, which leads to masking of symptoms and a delayed diagnosis.47,48 The most common initial misdiagnoses are chronic idiopathic PUD, chronic gastroesophageal reflux disease, and chronic diarrhea, but patients have also been diagnosed with irritable bowel syndrome, Crohn’s disease, celiac sprue, infectious diarrhea, giardiasis, lactose intolerance, biliary colic, chronic pancreatitis, and malabsorption syndrome.25,44
Patients with MEN-1 are more likely to present with severe esophageal disease, including Barrett’s esophagus.49 Diarrhea is a less common feature of ZES in MEN-1 patients.25 Otherwise, the clinical presentation is similar to that of sporadic gastrinoma. Despite the fact that approximately half of patients with MEN-1 develop gastrinomas, the diagnosis is still often missed or delayed. Because recognition of the syndrome can be challenging, only about 5% of MEN-1 patients receive the correct diagnosis on initial presentation.49
No unique staging systems for gastrinoma currently exist, and it is included in classification schemes that are applied to foregut/pancreatic NETs in general. However, even these grading and staging systems are not well established because of the rare incidence and relatively indolent course of NETs.50 In fact, the first system to classify pancreatic NETs was not proposed until 2002. Hochwald and colleagues51 reviewed 136 resected NETs (19 gastrinomas, 21 insulinomas, 5 VIPomas, and 2 glucagonomas, and 89 nonfunctional tumors), and they stratified the tumors into low- or intermediate-grade based on mitotic count and degree of necrosis. The next attempt at classifying NETs was published by the World Health Organization (WHO) in 2004. Based on tumor size, mitotic count, proliferation index, vascular invasion, perineural invasion, gross localization, and metastasis, they divided NETs into three categories: those with benign behavior, those with uncertain behavior, and well-differentiated endocrine carcinomas.52 In 2010, the WHO categories of NET were revised to well-differentiated (Grades 1 and 2) and poorly differentiated (Grade 3) NETs, on the basis of mitotic count and proliferative index alone.53
The first TNM staging system for foregut NETs was proposed in 2006 by the European Neuroendocrine Tumor Society (ENETS).54 This proposal separated foregut NETs into gastric, duodenal, and pancreatic locations, but did not differentiate according to tumor cell type or functionality.55 Four years later, the International Union for Cancer Control (UICC) developed another TNM staging system, which does not distinguish between pancreatic NET and exocrine pancreatic adenocarcinoma. This latter system has since been adopted by the WHO and the American Joint Cancer Committee (AJCC).53,54,56 The differences between the ENETS and the AJCC TNM systems are highlighted in Table 47-1. NETs arising in the duodenum are staged similarly in the ENETS and the AJCC systems.55,56 Both TNM systems have been validated, although some recent data suggests that the ENETS system is a more accurate prognostic tool.50,53,54,57
Comparison of TNM Staging Systems for Pancreatic Neuroendocrine Tumors from the European Neuroendocrine Tumor Society (ENETS) and the International Union for Cancer Control (UICC)/American Joint Cancer Committee (AJCC)/World Health Organization (WHO)
| Definitions | ENETS TNM (2006) | UICC/AJCC/WHO TNM (2010) |
|---|---|---|
| T Definition | ||
| T1 | Limited to the pancreas; <2 cm | |
| T2 | Limited to the pancreas; 2–4 cm | Limited to the pancreas; >2 cm |
| T3 | Limited to the pancreas, >4 cm or invading duodenum or bile duct | Extends beyond the pancreas, but without involvement of the celiac axis or superior mesenteric artery |
| T4 | Tumor invading adjacent organs (stomach, spleen, colon, adrenal gland) or the wall of large vessels (celiac axis or the superior mesenteric artery) | Tumor involves the celiac axis or the superior mesenteric artery (unresectable) |
| N Definition | ||
| N0 | No regional lymph node metastasis | No regional lymph node metastasis |
| N1 | Regional lymph node metastasis | Regional lymph node metastasis |
| M Definition | ||
| M0 | No distant metastasis | No distant metastasis |
| M1 | Distant metastasis | Distant metastasis |
| Stage Definition | ||
| Stage I |
|
|
| Stage II |
|
|
| Stage III |
|
|
| Stage IV |
|
|
Gastrinomas demonstrate aggressive behavior in approximately 25% of patients with sporadic ZES and in 15% of MEN-1 patients with ZES. The remaining patients have a more indolent course.25,26 Aggressive behavior is also associated with female gender, pancreatic primary tumors, a younger age at presentation, and higher fasting serum gastrin levels.58 Ten-year survival with the aggressive form is 30%, compared with 96% in patients with indolent tumors.25 Recurrence is common, and disease progression occurs in as many as 40% of patients, even after an R0 or R1 resection.2
The most important prognostic factor for survival in gastrinoma patients is the presence of liver metastases, with 10-year survival in patients with liver metastases ranging from 10% to 30%.1,3,26,58,59 In a prospective observational study of more than 200 gastrinoma patients at the NIH, Yu and colleagues reported that patients who did not have liver metastases had a 15-year survival of 93%. In contrast, 10-year survival was 68% for those who developed liver metastases during the study period and only 26% for those who presented with liver metastases at their initial evaluation.58 Resection of liver metastases appears to prolong survival (70% to 100% 5-year survival), but these data are somewhat difficult to interpret because most series include non-gastrin-secreting pancreatic NETs as well.2 Additionally, only 5% to 25% of patients with metastatic gastrinoma to the liver have resectable disease.1,60
Other poor prognostic factors include bone metastases, large size of primary tumor (>3 cm), ectopic ACTH production by the gastrinoma, poor tumor differentiation or rapid cell turnover (>2 mitoses/HPF and/or Ki67 proliferative index >4.85), local invasion, and inadequate control of gastric acid secretion.25,26,54,58 The prognostic value of lymph node metastases is less clear. In both the TNM staging systems described above, the presence of any lymph node metastasis is considered Stage IIB/III disease.55,56 When considering NETs in general, some authors have suggested that survival is decreased with lymph node metastases,54 while others claim there is no difference.61 Krampitz and colleagues59 did not show a difference in survival, but did demonstrate a shorter time to the development of liver metastases in the presence of lymph node metastases and therefore predicted there would be a decrease in disease-related survival with a sufficient follow-up interval. With regard to gastrinomas specifically, some have proposed that metastatic lymph node disease has no impact on survival.43,62 More recently, however, several small series have demonstrated that a systematic lymphadenectomy at the time of gastrinoma resection decreases recurrence rate and improves survival, thus suggesting that lymph node metastases do indeed adversely affect long-term survival.63,64
Interestingly, it has been proposed that widespread use of PPIs is actually worsening the prognosis of gastrinoma. In a single-institutional review of 108 patients with ZES diagnosed between 1948 and 1998, the patients were divided into four eras: initial recognition (1955 to 1965), increasing recognition (1966 to 1975), widespread application of gastrin radioimmunoassay (1976 to 1985), and widespread use of effective medical therapy (1986 to 1998). Although the number of patients seen per year remained constant, the 5-year survival was 45%, 74%, 90%, and 69%, and the disease-free survival was 0%, 4%, 29%, and 2% for each study period, respectively.48 While these observations have not been replicated by other groups to date, these authors suggest that the decline in both overall survival and disease-free survival in the modern era can be attributed to the masking of symptoms by effective medical therapy.
After surgical resection of gastrinoma, eugastrinemia is achieved in 30% to 50% of cases.3,47,65,66 In MEN-1 patients, this rate falls to 16% immediately postoperatively, and only 6% at 5 years.25,66 In patients with sporadic gastrinoma, however, there is some evidence that undergoing surgery will improve their outcomes regardless of biochemical cure.67 For the 30% to 50% who do achieve eugastrinemia, some small series have suggested that 90% remain disease-free after 3 years,68 but others report a recurrence rate as high as 40% by 10 years.2
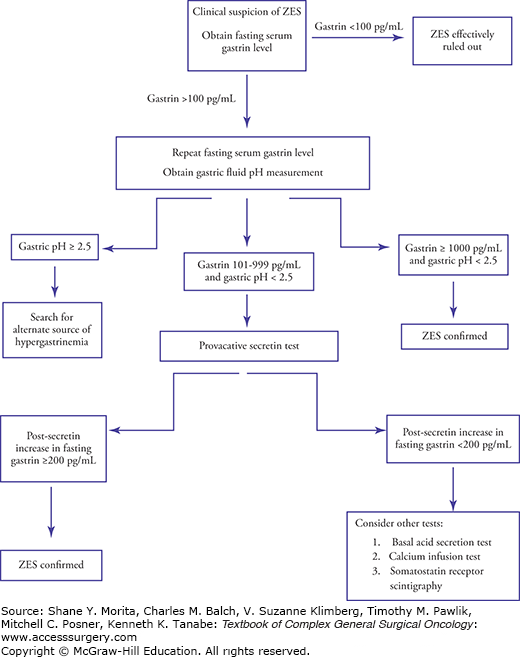
The initial diagnosis of gastrinoma is mainly a biochemical one, but it can be quite elusive. It requires the demonstration of inappropriate hypergastrinemia in the presence of gastric acid hypersecretion.38 Figure 47-2 is an algorithm to guide biochemical workup when ZES is clinically suspected. The first laboratory test to order is a fasting serum gastrin, which is elevated in 99% to 100% of gastrinoma patients.1,69 If this level is <100 pg/mL, gastrinoma is effectively ruled out. However, a fasting serum gastrin level >100 pg/mL requires further consideration because there are numerous conditions that result in hypergastrinemia.1,25,26,38,69 These can be categorized into appropriate hypergastrinemia (a normal physiologic response to hypochlorhydria or achlorhydria), inappropriate hypergastrinemia (elevated gastrin associated with gastric acid hypersecretion that does not respond to physiologic feedback inhibition), and spurious hypergastrinemia (caused by an inaccurate assay or a nonfasting patient) (Table 47-2).
Causes of Hypergastrinemia
|
|
|
Many causes of hypergastrinemia can be ruled out by history (i.e., previous gastric surgery, gastric outlet obstruction, and chronic renal failure), but excluding other etiologies requires a determination of the gastric pH. This can be performed by measuring pH of gastric aspirate obtained endoscopically or via nasogastric tube at the same time as a repeat fasting serum gastrin level. In patients whose gastric pH is >3.0, formal gastric secretion studies should be done to measure the rate of basal acid output (BAO).47 The combination of a fasting serum gastrin >1000 pg/mL and gastric pH<2 (or a BAO >15 mEq/h) is diagnostic for ZES.3,26,38,69 However, only one-third of patients with ZES have such markedly elevated gastrin levels; the other two-thirds have only moderate hypergastrinemia (100 to 999 pg/mL).3,25 In these individuals, further testing is required.
The provocative secretin test is used to distinguish ZES from other hypergastrinemia syndromes in the setting of moderate hypergastrinemia and low gastric pH. Under normal physiologic circumstances, secretin binds to gastrin-secreting antral G cells and somatostatin-secreting D cells. Somatostatin inhibits release of gastrin by the G cells, and therefore the serum gastrin level increases minimally, if at all, with secretin stimulation. In the setting of a gastrinoma, however, secretin (2 U/kg administered intravenously) stimulates both the gastrinoma cells and the G cells to release gastrin, and this exaggerated gastrin release is not overcome by the paracrine inhibition of somatostatin. Serum gastrin should be measured at 0, 2, 5, 10, 15, and 30 minutes after administration of secretin, and a paradoxical increase in serum gastrin levels (increase of >200 pg/mL) is positive for gastrinoma.2,47,69 Achlorhydria, including PPI therapy, can lead to a false-positive secretin stimulation test, but one would not expect a low gastric pH in these patients.25,47
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree