Gastric cancer is common and is a cause of severe morbidity and mortality. Early diagnosis can improve the chances of cure and prolong survival because prognosis is inversely related to the disease stage. Endoscopy plays an important role in diagnosis. Emerging adjunct technologies such as image-enhanced endoscopy and magnification endoscopy aid in early cancer detection. Endoscopic ultrasonography is an additional useful tool for preoperative staging. Endoscopy for screening, except for high-risk patients, and outside areas of high prevalence, remains controversial.
Gastric cancer is the fourth most common cancer worldwide, leading to more than 700,000 deaths annually, which is second only to lung cancer. Gastric cancer carries a poor prognosis, with 15% to 20% 5-year overall survival even if the disease is limited to the gastric wall. Outcomes are favorable if cancers and their precursors are detected early, and there is a wide window of opportunity for early detection because progression from early to advanced cancer is slow. However, being asymptomatic until an advanced stage, one-third of gastric cancers are metastatic at time of diagnosis.
This article reviews the role of endoscopy, which is the modality of choice for the diagnosis of gastric cancer. Morphologic classification, the use adjunct technologies that aid in early detection and staging, and screening are also discussed.
Classification and staging
Classification and staging of gastric cancer defines the disease and its behavior, dictates treatment decisions, and determines the prognosis. It also creates common grounds for communication among multiple care providers.
Gastric cancer is classified as early or advanced based on the distinct prognostic differences between these stages. Early gastric cancer (EGC) is limited to the gastric mucosa or submucosa irrespective of lymph node spread and regardless of clinical presentation. EGC undergoes a period of slow growth with estimated median disease duration of 37 months before progression into advanced cancer. In advanced cancer, tumor cells invade the muscularis propria and beyond. Patients with EGC tend to be younger and tend to have longer symptom duration.
The International Union Against Cancer (UICC) and the American Joint Committee on Cancer (AJCC) use the tumor node metastasis (TNM) system for clinical (c) or pathologic (p) staging of gastric cancer. Proximal gastric tumors whose midpoint is at or within, 5 cm from the gastroesophageal junction follow TNM staging for esophageal adenocarcinoma. Tumors located further distally, in the fundus, body, and antrum of the stomach, follow the gastric TNM staging.
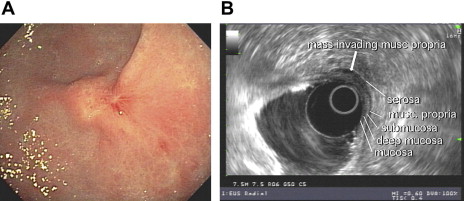
Depth of penetration of the primary tumor designates the T stage. The number of regional lymph nodes comprises the N stage, whereas distant (nonregional) lymph nodes, or peritoneal or other organ involvement, marks the M stage. EGC is a T1 category ( Table 1 ).
| Primary Tumor (T) | |
|---|---|
| TX | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| Tis | Carcinoma in situ: intraepithelial tumor without invasion of the lamina propria |
| T1 | Tumor invades lamina propria, muscularis mucosae, or submucosa |
| T1a | Tumor invades lamina propria or muscularis mucosae (T1m) |
| T1b | Tumor invades submucosa (T1sm) |
| T2 | Tumor invades muscularis propria |
| T3 | Tumor penetrates subserosal connective tissue without invasion of visceral peritoneum or adjacent structures |
| T4 | Tumor invades serosa (visceral peritoneum) or adjacent structures |
| T4o | Tumor invades serosa (visceral peritoneum) |
| T4b | Tumor invades adjacent structures |
| Regional Lymph Nodes (N) | |
|---|---|
| NX | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph nodes |
| N1 | Metastasis in 1–2 regional lymph nodes |
| N2 | Metastasis in 3–6 regional lymph nodes |
| N3 | Metastasis in 7 or more regional lymph nodes |
| N3a | Metastasis in 7–15 regional lymph nodes |
| NBb | Metastasis in 16 or more regional lymph nodes |
| Distant Metastasis | |
|---|---|
| M0 | No distant metastasis |
| Ml | Distant metastasis |
The Lauren histologic classification into intestinal and diffuse types is traditionally used in the Western world. The intestinal type, as the name implies, is characterized by the formation of glandular structures (intestinal metaplasia) comprising well-differentiated columnar epithelial cells. The diffuse type is characterized by pangastric infiltration by poorly cohesive clusters or solitary mucin-rich (signet ring) cells. Transmural extension through lymphatic invasion can lead to gastric wall thickening without causing a discrete mass (linitis plastica). Intestinal-type gastric cancer more commonly involves the distal stomach. It is closely linked to environmental ( Helicobacter pylori ) and dietary exposure and is declining. Diffuse-type gastric cancer tends to occur at a younger age and carries a worse prognosis.
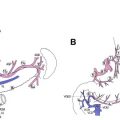
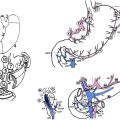
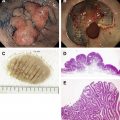
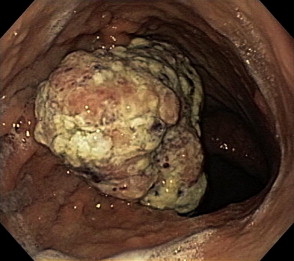
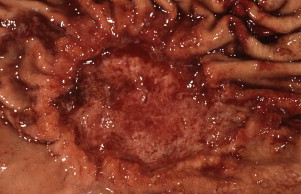
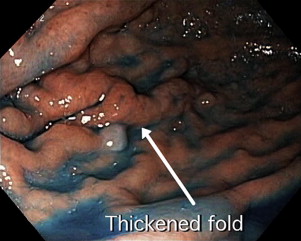
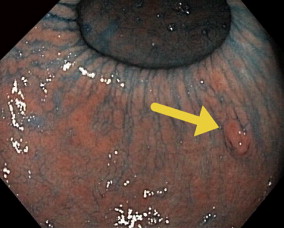
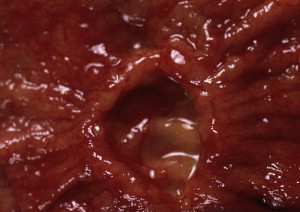
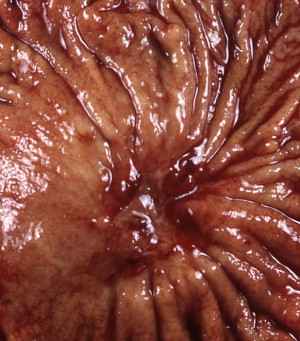
Gastric cancer can exhibit different morphologic growth patterns. Advanced cancers are classified according to Borrmann ( Table 2 ) into polypoid (type I) ( Fig. 1 ), fungating (type II) ( Fig. 2 ), ulcerated (type III) ( Fig. 3 ), or infiltrative (type IV) ( Fig. 4 ) cancers. The Paris endoscopic classification ( Table 3 ) for superficial neoplastic lesions (type 0) incorporates the macroscopic classification system of the Japanese Gastric Cancer Association. It distinguishes the polypoid type 0–I (subdivided into p, pedunculated; s, sessile), the superficial (or nonpolypoid) type 0–II (a, elevated [ Fig. 5 ]; b, flat; c, depressed) and the excavated type 0–III ( Fig. 6 ) with 0–IIa and 0–IIc being the most common variants. Type 0–IIa lesions have a thickness equal to or less than twice that of the normal mucosa, whereas type 0–I lesions have a thickness that exceeds twice that of the normal mucosa. If a single lesion contains a combined pattern, the one occupying the greatest area is recorded first ( Fig. 7 ).
Endoscopic detection
Gastric cancer is usually asymptomatic until an advanced stage. If symptoms occur, they are similar to dyspepsia or peptic ulcer disease. Endoscopy is the procedure of choice for diagnosis. The accuracy of endoscopy in the detection and diagnosis of EGC is reported to range between 90% and 96%. Endoscopy allows for tumor localization, morphologic characterization, and tissue diagnosis through biopsy. The American Gastroenterological Association recommends an upper endoscopy be performed in patients with new-onset dyspepsia who are older than 55 years as well as in patients with alarm symptoms (such as weight loss, recurrent vomiting, gastrointestinal [GI] bleeding, or family history of cancer). The use of acid antisecretory therapy can mask symptoms and may delay early diagnosis. EGC can assume different morphologic appearances on endoscopy, such as subtle polypoid protrusions, superficial plaques, or depressions. Ulcerated malignancies carry a higher risk of deep invasion and lymph node metastases compared with flat lesions. The distinction between ulcerating cancer and benign peptic ulcers may not always be obvious When a suspicious or nonhealing ulcer is encountered, 6 to 8 biopsy specimens from the edges and base of the ulcer are recommended. This number of specimens provides a 98% sensitivity to detect malignancy. Brush cytology can add a little to the sensitivity. Concerns about false-negative histology, which can approach 5%, have been raised, and follow-up endoscopies have been advocated for all gastric ulcers to document healing. However, studies revealed that endoscopic follow-up until complete healing yielded few malignancies and even fewer at early stages. Because of the high sensitivity of multiple, adequately obtained biopsies, the usefulness of repeat endoscopy remains in question, especially if an experienced endoscopist deems the ulcer to be benign.
In the absence of a consensus, cautious practice is warranted in patients with gastric ulcers and increased pretest prevalence for gastric cancer. These high-risk patients include those with persistent upper GI symptoms despite adequate antisecretory therapy, those older than 50 years of age, those with family history of gastric cancer, or those who belong to certain ethnic groups in endemic areas (eg, Asians). Absence of nonsteroidal antiinflammatory drug (NSAID) use should increase concern. Protracted history of peptic ulcer disease and concomitant presence of duodenal ulcers makes malignancy less likely.
The main method for discriminating malignant from benign ulcers is adequate biopsy. However, there are several criteria to macroscopically distinguish between benign and malignant ulcers based on endoscopic or radiologic findings. Benign gastric ulcers tend to have regular shapes (linear, round, or oval); even bases; smooth, sharply demarcated symmetric borders; and surrounding gastric folds that converge toward the ulcer. Malignant gastric ulcers have geographic shapes; an uneven (sometimes shallow or necrotic) base; asymmetric elevated edges with rigid, thick, irregular borders; and disrupted periulcer folds near the crater edge and/or clubbed or fused folds. Malignant ulcers may be associated with a mass lesion and the surrounding gastric mucosa may appear abnormal. Although both benign and malignant ulcers can vary in size and can occur in multiplicity and in any location within the stomach, ulcers in the proximal half of the greater curvature and fundus should be suspicious for malignancy. Healed scars from benign gastric ulcers appear as collection of gastric folds converging toward a central pit. An underlying malignancy should be suspected if the scar is irregular, has nodular edges, or the radiating folds are amputated.
Endoscopic detection
Gastric cancer is usually asymptomatic until an advanced stage. If symptoms occur, they are similar to dyspepsia or peptic ulcer disease. Endoscopy is the procedure of choice for diagnosis. The accuracy of endoscopy in the detection and diagnosis of EGC is reported to range between 90% and 96%. Endoscopy allows for tumor localization, morphologic characterization, and tissue diagnosis through biopsy. The American Gastroenterological Association recommends an upper endoscopy be performed in patients with new-onset dyspepsia who are older than 55 years as well as in patients with alarm symptoms (such as weight loss, recurrent vomiting, gastrointestinal [GI] bleeding, or family history of cancer). The use of acid antisecretory therapy can mask symptoms and may delay early diagnosis. EGC can assume different morphologic appearances on endoscopy, such as subtle polypoid protrusions, superficial plaques, or depressions. Ulcerated malignancies carry a higher risk of deep invasion and lymph node metastases compared with flat lesions. The distinction between ulcerating cancer and benign peptic ulcers may not always be obvious When a suspicious or nonhealing ulcer is encountered, 6 to 8 biopsy specimens from the edges and base of the ulcer are recommended. This number of specimens provides a 98% sensitivity to detect malignancy. Brush cytology can add a little to the sensitivity. Concerns about false-negative histology, which can approach 5%, have been raised, and follow-up endoscopies have been advocated for all gastric ulcers to document healing. However, studies revealed that endoscopic follow-up until complete healing yielded few malignancies and even fewer at early stages. Because of the high sensitivity of multiple, adequately obtained biopsies, the usefulness of repeat endoscopy remains in question, especially if an experienced endoscopist deems the ulcer to be benign.
In the absence of a consensus, cautious practice is warranted in patients with gastric ulcers and increased pretest prevalence for gastric cancer. These high-risk patients include those with persistent upper GI symptoms despite adequate antisecretory therapy, those older than 50 years of age, those with family history of gastric cancer, or those who belong to certain ethnic groups in endemic areas (eg, Asians). Absence of nonsteroidal antiinflammatory drug (NSAID) use should increase concern. Protracted history of peptic ulcer disease and concomitant presence of duodenal ulcers makes malignancy less likely.
The main method for discriminating malignant from benign ulcers is adequate biopsy. However, there are several criteria to macroscopically distinguish between benign and malignant ulcers based on endoscopic or radiologic findings. Benign gastric ulcers tend to have regular shapes (linear, round, or oval); even bases; smooth, sharply demarcated symmetric borders; and surrounding gastric folds that converge toward the ulcer. Malignant gastric ulcers have geographic shapes; an uneven (sometimes shallow or necrotic) base; asymmetric elevated edges with rigid, thick, irregular borders; and disrupted periulcer folds near the crater edge and/or clubbed or fused folds. Malignant ulcers may be associated with a mass lesion and the surrounding gastric mucosa may appear abnormal. Although both benign and malignant ulcers can vary in size and can occur in multiplicity and in any location within the stomach, ulcers in the proximal half of the greater curvature and fundus should be suspicious for malignancy. Healed scars from benign gastric ulcers appear as collection of gastric folds converging toward a central pit. An underlying malignancy should be suspected if the scar is irregular, has nodular edges, or the radiating folds are amputated.
Endoscopic image–enhancing technologies
EGC, which can appear as a flat lesion or superficial depression, can be endoscopically indistinguishable from mucosal inflammation or noncancerous erosions. Advancements in endoscope technologies improve the ability to detect and characterize gastric cancer. Additional image-enhancing methods and mucosal staining techniques are used to reduce the miss rate and to delineate margins facilitating endoscopic treatments such as endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD).
The introduction of high-definition endoscopes and the use of narrow-band imaging have yielded higher quality images with improved resolution and contrast. Magnification endoscopy, with its ability to evaluate fine mucosal surface patterns and microvasculature in real-time, may aid in the initial evaluation of lesions before histologic diagnosis. Endocytoscopy and confocal laser endomicroscopy, with 1000-fold magnification, permit in vivo high-resolution evaluation of the GI mucosa at the cellular level, providing what has been termed optical biopsies. Coupled with the instillation of chemicals such as acetic acid (enhanced magnification) or topical application of pigments or stains (magnification chromoendoscopy), the images can be further improved with visualization of fine surface patterns not seen with standard endoscopy. The following stains are commonly used to enhance mucosal contrast (chromoendoscopy): methylene blue (methylthionine chloride) is a carbon-based vital stain that selectively and reversibly binds to actively absorbing epithelium, such as gastric intestinal metaplasia, and not to normal columnar gastric epithelium. Indigo carmine is a contrast stain that highlights fine mucosal morphology, aiding in recognition of subtle topographic changes. The instillation of acetic acid causes reversible alteration in tertiary structures of cellular proteins. The acetic acid–induced whitening of the columnar mucosa produces an enhanced view of mucosal structures. Narrow-band imaging (NBI) is a virtual form of chromoendoscopy. Using a combination of 2 short wavelengths (415 nm and 540 nm) within a narrow bandwidth, NBI is capable of enhancing mucosal architecture and microvasculature. Autofluorescence imaging videoendoscopy (AFI), which uses the autofluorescence of endogenous fluorophores (eg, collagen, nicotinamide, flavin, porphyrins, and adenine dinucleotide) from light excitation, produces real-time pseudocolor images that can distinguish between (green) normal and dysplastic/neoplastic tissue (magenta purple).
These modalities, alone or in combination, have also been used in the identification of premalignant lesions within the intestinal-type gastric adenocarcinoma cascade (eg, intestinal metaplasia, dysplasia). However, most of the studies involving magnification endoscopy (enhanced or not) and chromoendoscopy that suggest improved discriminatory ability between benign, premalignant, and malignant lesions, remain descriptive.
Using magnification endoscopy (ME), Yao and colleagues described 3 characteristic findings in differentiated gastric adenocarcinoma, including disappearance of regular subepithelial capillary network pattern, the presence of an irregular microvascular pattern, and the presence of a demarcation line. Nonstructural appearance caused by the lack of a normal tubular pattern suggests that the cancer involves the submucosal layer. Tajiri and colleagues described an irregular coarse mucosal surface pattern in elevated-type carcinomas and a finer pit pattern or destruction/disappearance of the mucosal microstructure and abnormal capillary vessels in depressed-type carcinomas. Otsuka and colleagues classified the surface structure of EGC into small regular pattern of sulci and ridges, irregular pattern of sulci and ridges, or lack of visible structure. Microvasculature of cancerous lesions was described as either of varied caliber or minute and irregular.
With dynamic enhanced ME (EME), Yagi and colleagues noticed that, compared with noncancerous gastric lesions, carcinomas returned faster to baseline color appearance after acetowhitening, further enhancing the margins between neoplastic and nonneoplastic tissue. Tanaka and colleagues, using EME, described 5 surface patterns of gastric tumors and surrounding mucosa: small round pits of uniform size (type I), slitlike pits (type II), and fine villous or gyrus pattern (type III). Type IV is an irregular arrangement and sizes of the aforementioned patterns, and type V is a destructive pattern. All depressed-type EGCs were classified as type IV or V, whereas elevated-type cancers were classified as either type III or IV. The presence of types IV to V strongly correlated with underlying malignancy (sensitivity 100%, specificity 89.7%).
ME-NBI is able to differentiate gastric tumors from background nonmalignant mucosa. In a study of 136 patients, Dinis-Ribeiro and colleagues, using magnification chromoendoscopy with methylene blue staining, classified gastric lesions based on mucosal and pit pattern with a diagnostic sensitivity and specificity of 76% and 87% respectively for intestinal metaplasia and 98% and 81% respectively for dysplasia. The reproducibility of this classification was further validated by Areia and colleagues. Using ME-NBI, Sumiyama and colleagues demarcated EGC based on differences in capillary structures. Kato and colleagues showed that, in patients who had lesions previously identified with white-light endoscopy (WLE), ME-NBI is superior to WLE in diagnosis of gastric cancer when the diagnostic triad of the disappearance of fine mucosal structure, microvascular dilation, and heterogeneity is used. Triad-based diagnosis by ME-NBI had sensitivity and specificity of 92.9% and 94.7% respectively, compared with 43% sensitivity and 61% specificity of WLE. In a prospective study involving 57 gastric depressive lesions smaller than 10 mm, Ezoe and colleagues found that the diagnostic accuracy was significantly better for ME-NBI than for ME using white-light imaging (79% vs 44%) as was the sensitivity (70% vs 33%), with no significant difference in specificity. Kadowaki and colleagues compared 4 methods of ME: conventional ME (CME), EME, ME-NBI, and EME-NBI. Despite the small number of enrolled patients (n = 37) EME-NBI was significantly better than CME alone in identifying EGC demarcation, followed by EME or ME-NBI.
Kiyotoki and colleagues found ME-NBI to be significantly more accurate than indigo carmine chromoendoscopy (97% vs 78% respectively) in identifying tumor margins in patients undergoing ESD for EGC.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree