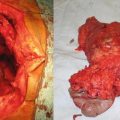
Fig. 9.1
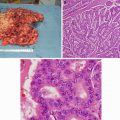
EGD picture of a 51-year-old man with a large gastric fundus adenocarcinoma (Courtesy of Quyen D. Chu, MD, MBA, FACS)
While an EGD confirms the pathologic diagnosis in most cases, it is important to recognize cases that may lack an obvious mucosal component. These tumors often result in poor gastric distensibility (linitis plastica) secondary to diffuse submucosal infiltration and require deeper biopsies for diagnosis. Typical staging studies include contrast-enhanced computed tomography (CT) of the chest, abdomen, and pelvis (Fig. 9.2). While this modality provides excellent definition of nodal and visceral metastases, it has low sensitivity for identifying metastases to the peritoneum. The presence of distant metastases on CT imaging essentially precludes the need for any additional staging procedures. For those patients with radiographically localized disease, EUS can provide useful information about the depth of invasion, especially in early gastric cancer [9]. Its role in predicting nodal involvement, however, is less reliable, especially when the nodal stations are further away from the probe.
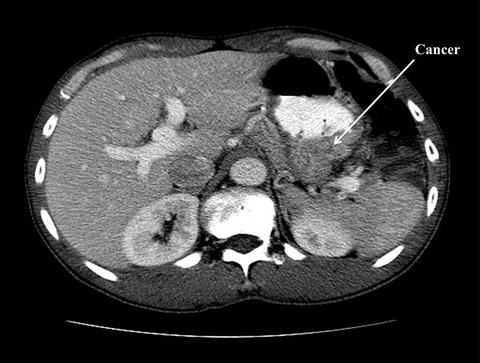

Fig. 9.2
CT scan of the same gentleman who has a large gastric fundus adenocarcinoma. There was no evidence of nodal disease on CT scan (Courtesy of Quyen D. Chu, MD, MBA, FACS)
The role of 18-fluoro-deoxyglucose positron emission tomography (FDG-PET) in the staging of gastric cancer is evolving (Fig. 9.3). While it can serve as an adjunct to CT imaging in the detection of occult metastatic disease [10], its use as a stand-alone staging tool is limited by a high false-negative rate in tumors of low metabolic uptake and those that have a low expression of SLC2A1, a transmembrane transporter of FDG [11, 12].
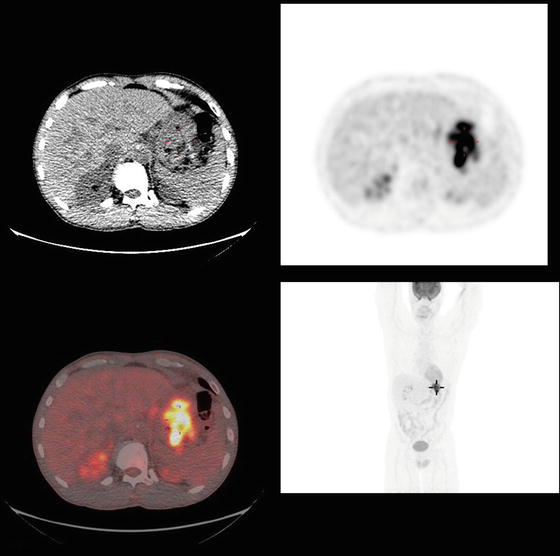

Fig. 9.3
PET/CT of the same gentleman who has a large gastric fundus adenocarcinoma. There was evidence of markedly intense FDG uptake in an area of gastric fundus extending to greater curvature consistent with aggressive neoplasm. There was no evidence of locoregional or distant disease. The patient underwent a total gastrectomy and splenectomy (due to tumor extending into the splenic hilum) with a D2 dissection. Final pathology demonstrated a moderately differentiated T4 adenocarcinoma (9 cm maximal diameter) with 11 out of 26 positive lymph nodes. The closest margin was 5 cm from the tumor. Adjuvant chemoradiation was given (Courtesy of Quyen D. Chu, MD, MBA, FACS)
A diagnostic laparoscopy (DL) with lavage to exclude peritoneal metastases is utilized at many institutions prior to surgery with curative intent or the delivery of planned neoadjuvant therapy. A positive laparoscopy, including positive cytology from washings, is considered as M1 disease, which portends a poor prognosis and alters the treatment plan in a substantial number of patients, including avoidance of a laparotomy [13, 14]. DL is useful in situations where the patient is at risk of harboring distant disease (i.e., T3 or N1 disease identified on preoperative imaging). However, DL is limited in detecting disease in the perigastric lymph nodes as well as small intraparenchymal liver metastases.
Adenocarcinoma of the gastroesophageal junction (GEJ) has been considered as either esophageal or gastric cancer. In 1987, Siewert et al. classified these tumors into three types [15]. Type 1 tumors are those with the epicenter 1–5 cm proximal to the GEJ (adenocarcinoma of the distal esophagus), type II are those with epicenter 1 cm proximal to the GEJ and 2 cm distal to GEJ (true cardiac cancer), and type III are those with the epicenter 2–5 cm distal to the GEJ (inferior cardiac cancer) (Fig. 9.4).
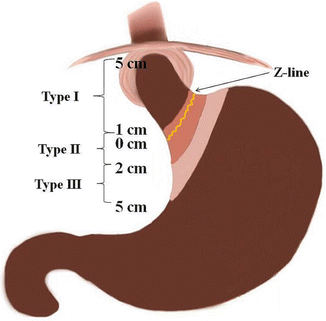

Fig. 9.4
Siewert classification of adenocarcinoma of the gastroesophageal junction: type I (esophageal adenocarcinoma) is from 5 to 1 cm above the Z line, type II (cardia cancer) is from 1 cm over to 2 cm below the Z line, and type III is from 2 cm below to 5 cm below the Z line (Courtesy of Quyen D. Chu, MD, MBA, FACS)
There are two staging systems that are widely used in clinical practice and research, the American Joint Committee on Cancer (AJCC/TNM) staging system according to the 7th edition (Table 9.1) and the Japanese staging system. The Japanese staging system is based on the anatomic involvement of the nodal stations [17]. The AJCC/TNM staging directly affects the potential success of an R0 resection [8].
Table 9.1
American Joint Committee on Cancer (AJCC) TNM staging for stomach carcinomas (7th edition)
Primary tumor (T) | |
|---|---|
TX | Primary tumor cannot be assessed |
T0 | No evidence of primary tumor |
Tis | Carcinoma in situ: intraepithelial tumor without invasion of the lamina propria |
T1 | Tumor invades lamina propria, muscularis mucosae, or submucosa |
T1a | Tumor invades lamina propria or muscularis mucosae |
T1b | Tumor invades submucosa |
T2 | Tumor invades muscularis propria* |
T3 | Tumor penetrates subserosal connective tissue without invasion of visceral peritoneum or adjacent structures **, *** |
T4 | Tumor invades serosa (visceral peritoneum) or adjacent structures**, *** |
T4a | Tumor invades serosa (visceral peritoneum) |
T4b | Tumor invades adjacent structures |
*Note: A tumor may penetrate the muscularis propria with extension into the gastrocolic or gastrohepatic ligaments, or into the greater or lesser omentum, without perforation of the visceral peritoneum covering these structures. In this case, the tumor is classified T3. If there is perforation of the visceral peritoneum covering the gastric ligaments or the omentum, the tumor should be classified T4 | |
**The adjacent structures of the stomach include the spleen, transverse colon, liver, diaphragm, pancreas, abdominal wall, adrenal gland, kidney, small intestine, and retroperitoneum | |
***Intramural extension to the duodenum or esophagus is classified by the depth of the greatest invasion in any of these sites, including the stomach | |
Anatomic stage/prognostic groups | |||
|---|---|---|---|
Group | T | N | M |
Stage 0 | Tis | N0 | M0 |
Stage IA | T1 | N0 | M0 |
Stage IB | T2 | N0 | M0 |
T1 | N1 | M0 | |
Stage IIA | T3 | N0 | M0 |
T2 | N1 | M0 | |
T1 | N2 | M0 | |
Stage IIB | T4a | N0 | M0 |
T3 | N1 | M0 | |
T2 | N2 | M0 | |
T1 | N3 | M0 | |
Stage IIIA | T4a | N1 | M0 |
T3 | N2 | M0 | |
T2 | N3 | M0 | |
Stage IIIB | T4b | N0 | M0 |
T4b | N1 | M0 | |
T4a | N2 | M0 | |
T3 | N3 | M0 | |
Stage IIIC | T4b | N2 | M0 |
T4b | N3 | M0 | |
T4a | N3 | M0 | |
Stage IV | Any T | Any N | M1 |
Regional lymph nodes (N)† | |
|---|---|
NX | Regional lymph nodes cannot be assessed |
N0 | No regional lymph nodes metastasis* |
N1 | Metastasis in 1–2 regional lymph nodes |
N2 | Metastasis in 3–6 regional lymph nodes |
N3 | Metastasis in ≥ 7 regional lymph nodes |
N3a | Metastasis in 7–15 regional lymph nodes |
N3b | Metastasis in ≥ 16 regional lymph nodes |
†Retropancreatic, para-aortic, portal, retroperitoneal, and mesenteric nodes are considered M1-disease. | |
*A designation of pN0 should be used if all examined lymph nodes are negative, regardless of the total number removed and examined. | |
Distant metastasis (M) | |
|---|---|
M0 | No distant metastasis |
M1 | Distant metastasis |
In 2010, the AJCC made three important changes to the staging of gastric cancer. First, only tumors arising more than 5 cm distal to the gastroesophageal junction (GEJ) would be staged as gastric cancers; those at the GEJ and those within 5 cm of the GEJ with involvement of the GEJ (Siewert types I–III GEJ tumors) would be classified and staged as esophageal carcinoma. Siewert type III is considered gastric cancer only if the esophagus is not infiltrated. Secondly, the T3 category was changed from “invading the serosa” to “invading through the muscle into the subserosal connective tissue.” Tumors invading the serosa are now classified as T4 along with invasion into adjacent structures. This second change was implemented in an effort to align the staging of gastric cancers with tumors of the rest of the gastrointestinal tract (esophagus, small and large intestine). Thirdly, the T1 stage was subdivided into T1a (lamina propria or muscularis mucosa confined) and T1b (submucosal involvement) for data collection purposes [18].
Superior outcomes among the Japanese surgeons have been attributed to many reasons, one of which is the Will Rogers phenomenon. Will Rogers was a comedian who was believed to have made a comment about the plight of people during the 1930s Great Depression. He commented that “when the Okies left Oklahoma and moved to California, they raised the average intelligence level in both states.” How this applies to gastric staging is that when Japanese surgeons perform extended nodal dissection, they tend to more accurately stage their patients than surgeons from the USA, the majority of whom do not perform extended node dissection and therefore potentially understage their patients. Thus, patients who are stage II cancers in the USA may actually be stage III (stage migration), which may account for a lower survival in the USA than the Japanese data, stage for stage. This phenomenon is referred to as stage migration and suggests that the differences in outcomes between the two groups of surgeons were not due to more surgery (i.e., more extensive nodal dissection), but rather due to more accurate staging.
Treatment of Early Gastric Cancer and Local Control
Tumors confined to the mucosa and submucosa (T1) are referred to as early gastric cancers (EGC) regardless of lymph node status. Overall, these patients do very well, experiencing cure rates exceeding 80–90 % after surgery alone. The number of lymph node metastasis is an important prognostic factor. In a report from Japan, the 5-year survival rate was intimately linked to nodal disease burden, which decreases from 85 % for N0 disease to 61 % for N1 involvement, 31 % for N2 disease, 10 % for N3 disease, and only 2 % for N4 disease [19].
Despite the changes in incidence, screening, and diagnosis, the paradigm that guides operative management for gastric cancer has not changed over the last century – complete surgical resection is the only potentially curative treatment for localized disease. An important change occurred in the early 1990s when reports from large series from Japan showed the efficacy of using local resection techniques such as an endoscopic mucosal resection (EMR) without a lymphadenectomy or gastrectomy for a select group of patients who were at very low risk of having nodal metastases. The proposed selection criteria include: (1) the tumor is small (≤3 cm in diameter), (2) well differentiated and without lymphovascular invasion, and (3) superficially elevated and/or depressed but without ulceration or definitive submucosal invasion [20, 21]. Given the low likelihood of identifying such lesions in a Western population, its applicability to this cohort appears to be limited. Furthermore, long-term outcome with EMR is lacking. Thus, EMR should only be performed under the auspice of a clinical trial or be limited to medical centers that are highly experienced with such a technique.
If the tumor has spread into the submucosa, the incidence of lymph node metastasis increases, and more definitive surgery (i.e., gastrectomy) must be performed to ensure an R0 resection. A subject of great controversy in the past was whether all gastric cancer patients should have a total gastrectomy or a subtotal gastrectomy. Two prospective randomized trials, one from France and a second from Italy conducted over two decades ago, concluded the following: both procedures have similar 5-year survival rates, but a subtotal gastrectomy is a technically easier operation, is associated with less perioperative morbidity and mortality, allows for better postoperative nutritional status and quality of life, and hence is preferred over total gastrectomy, when feasible [22, 23]. Therefore, our current practice is to perform a subtotal gastrectomy for tumors of the distal stomach and body (Fig. 9.5a–b), depending on the tumor size and margin status and a total gastrectomy for most cancers of the proximal stomach and large tumors in the fundus. A Billroth II gastrojejunostomy (BII) is the preferred method of reconstruction with a caveat that the afferent limb should not be longer than 25 cm in order to avoid the acute afferent loop syndrome (ALS), a potentially serious condition that usually requires emergent surgical intervention (Fig. 9.5a).
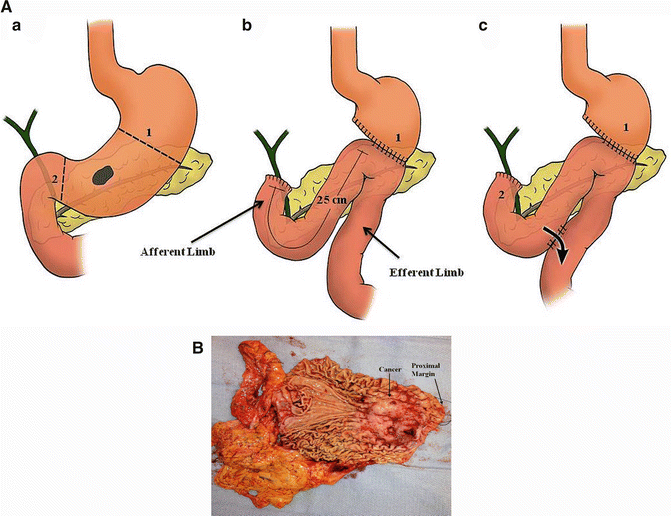

Fig. 9.5
Partial gastrectomy with a Billroth II (BII) gastrojejunostomy reconstruction. The “classic” BII gastrojejunostomy reconstruction is shown in Panel B. Panel C represents a BII gastrojejunostomy with a Braun enteroenterostomy (a). Afferent loop syndrome may occur if the afferent limb is made too long (>25 cm). In some instances, a patient may have prolonged gastroparesis following a subtotal gastrectomy and a BII reconstruction. A Braun enteroenterostomy can be used to address these complications and should be created approximately 25 cm distal to the BII gastrojejunostomy to divert contents of the afferent limb (bile) from the stomach. Figure 9.5b shows a large distal gastric adenocarcinoma that was resected with negative margins ((a, b): Courtesy of Quyen D. Chu, MD, MBA, FACS)
As an aside, patients who underwent a subtotal gastrectomy with a BII reconstruction may have a number of gastric motility disorders postoperatively. Two worth mentioning include gastroparesis and afferent loop syndrome. Symptoms for both include upper abdominal pain, nausea and vomiting, early satiety, and anorexia, although ALS tends to present with an acute abdomen. Patients are unable to tolerate per oral intake and require long-term nasogastric tube resulting in a prolonged postoperative recovery time and hospital stays. The causes of postoperative gastroparesis are unclear but may be related to damage of the gastric electrical pacemaker, gastrointestinal dysfunction, and vagus resection following subtotal gastrectomy [24]. As mentioned above, ALS is due to an obstruction of the afferent limb and is mainly due to technical errors (i.e., creation of a long afferent limb), but also due to twisting or kinking of the afferent limb or swelling at the anastomosis of the afferent limb to the stomach. Regardless, the solution to the above two conditions is to create a Braun enteroenterostomy (Fig. 9.5a), either at the time of the initial operation or when these complications occur. The Braun enteroenterostomy helps to divert contents of the afferent limb (bile) to the distal bowel.
For cancers in the proximal stomach, a total gastrectomy with a Roux-en-Y reconstruction is performed (Fig. 9.6), although a subtotal gastrectomy can still be considered if a negative margin can be achieved. The distance between the esophagojejunostomy anastomoses and the jejunojejunostomy anastomoses should be 40–60 cm in order to avoid symptomatic bile reflux.
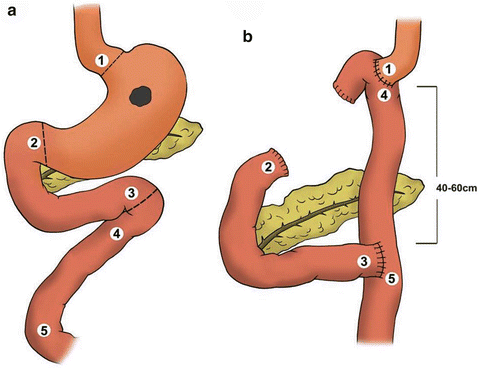

Fig. 9.6
Total gastrectomy with a Roux-En-Y esophagojejunostomy reconstruction. Note that the proximal jejunal “Y” limb should be anastomosed to the distal jejunal “Roux” limb approximately 40–60 cm from the esophagojejunostomy anastomoses to avoid symptomatic bile reflux (Courtesy of Quyen D. Chu, MD, MBA, FACS)
For the majority of gastric cancers, 5 cm is considered a safe proximal margin. The presence of a microscopic positive margin should be managed in the context of the extent of disease being treated. In patients without significant lymph node metastasis (≤5 positive lymph nodes out of 15 pathologically examined), survival is significantly decreased by leaving a positive margin. However, if patients that have five or more positive lymph nodes, the presence of a positive margin will have little influence on disease-related survival [25]. This brings us to our next consideration in the operative management of gastric tumors – regional control and the extent of lymph node resection.
Regional Control
In order to accurately depict a tumor as N0 within the AJCC/TNM classification, a minimum of 15 lymph nodes is required to be examined by pathology. The Japanese Research Society for the Study of Gastric Cancer has defined a standard for the extent of lymph node dissection and labeled them D1-4 (Fig. 9.7). D-dissection should be distinguished from R-resection; D-dissection describes the extent of lymphadenectomy, while R-resection describes the degree of residual tumor left behind after resection. An R0 resection is a complete resection without evidence of gross or microscopic disease; an R1 is one that has no gross disease, but has evidence of microscopic residual disease, and an R2 is one that has gross residual disease.
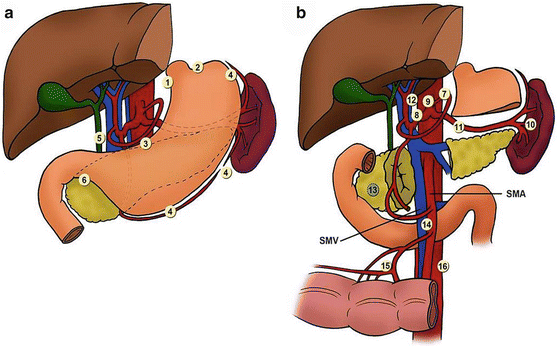

Fig. 9.7
Gastric cancer nodal stations: station 1 right paracardial; station 2 left paracardial; station 3 lesser curvature of the stomach, along left and right gastric arteries; station 4 greater curvature of the stomach, along left and right gastroepiploic arteries and short gastric arteries; station 5 suprapyloric, along right gastric artery; station 6 infrapyloric, along right gastroepiploic artery; station 7 trunk of left gastric artery; station 8 common hepatic artery; station 9 celiac artery; station 10 splenic hilum; station 11 splenic artery; station 12 hepatoduodenal ligament, along proper hepatic artery, common bile duct, and portal vein; station 13 posterior surface of pancreatic head; station 14 superior mesenteric vein; station 15 middle colic vessels; station 16 para-aortic. D1 dissection: stations 1–6; D2 dissection: stations 1–12 (Courtesy of Quyen D. Chu, MD, MBA, FACS)
A D1 dissection includes a subtotal or total gastrectomy and removal of only the perigastric nodes along the lesser and greater curvature of the stomach (Stations 1–6). A D2 dissection adds the removal of nodes along the left gastric artery, common hepatic artery, celiac trunk, and splenic artery and hilum (Stations 7–12). With some proximal T4 tumors, resection of the spleen and pancreatic tail is necessary to achieve adequate nodal clearance along the splenic artery and hilum, but in most cases the spleen and distal pancreas can and should be preserved (Fig. 9.8). Given the distinction between D-dissection and R-resection, one can see how it is possible to have an R1 resection (microscopic disease) with a D2 dissection.
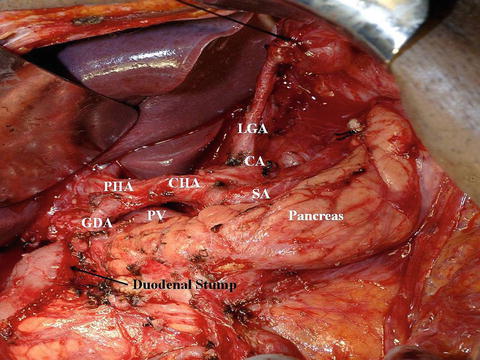

Fig. 9.8
Intraoperative picture of a modified D2 dissection for distal gastric cancer. The distal pancreas and spleen were preserved. LGA left gastric artery, CA celiac axis, SA splenic artery, CHA common hepatic artery, PV portal vein, PHA proper hepatic artery, GDA gastroduodenal artery (Courtesy of Quyen D. Chu, MD, MBA, FACS)
Prophylactic distal pancreatectomy and splenectomy should not be routinely performed; a splenectomy should only be done when the hilum is involved. D3 and D4 resections involve resecting more distant and periaortic nodes, and recent data has shown that this extent of lymphadenectomy does not improve survival over a D2 dissection [26, 27].
Just as total gastrectomy (in comparison to subtotal) is associated with greater postoperative morbidity and mortality, so can a more extensive lymph node dissection [25]. A randomized Dutch trial of 1,078 patients with gastric cancer (711 patients treated with curative intent), for which the majority had pathologic T1 to T3 tumors, found no long-term overall survival benefit from an extended D2 versus conventional D1 lymph node dissection. It was thought that the higher postoperative mortality rate associated with a D2 dissection likely offsets the long-term survival advantage with a D2 dissection [28]. In the final report from this landmark trial, which was exemplified by the rigorous quality control including training of Dutch surgeons by an expert counterpart from Japan in performing a D2 dissection, the overall survival for patients undergoing a D1 dissection was 30 % at 11 years compared to 35 % for those who underwent a D2 dissection (P = 0.53) [26]. However, when the investigators stratified patients postoperatively by lymph node stage, those with N2 disease who had a D2 resection had a statistically significant improvement in 11-year survival rates of 21 % compared with 0 % in the D1 group. At a median follow-up of 15 years, there was a lower locoregional recurrence rate in the D2 lymphadenectomy group than the D1 group, though this group had a higher rate of postoperative morbidity, mortality, and reoperation [29]. A meta-analysis of 12 randomized trials comparing D1 and D2 demonstrated no significant difference in OS (HR = 0.92, 95 % CI, 0.77–1.10, P = 0.36), but a subgroup analysis of patients who underwent a D2 dissection without a splenectomy and/or pancreatectomy demonstrated a trend towards OS benefit [30]. Therefore, D2 resection is a reasonable approach when the likelihood of the patient having locally advanced nodal disease exists. In Japan, a D2 dissection is considered a routine practice. However, D2 should only be performed by experienced and trained surgeons. To complete the operation, a feeding jejunostomy should be considered for postoperative feeding.
Laparoscopic techniques have become an integral part of surgical practice over the past several decades. For gastric cancer, multiple retrospective studies have reported the advantages of laparoscopic gastrectomy (LG) over open gastrectomy (OG) [31–34]. A recent meta-analysis of 15 non-randomized comparative studies has also shown that although LG had a longer operative time than OG, it was associated with lower intraoperative blood loss, overall complication rate, fewer wound-related complications, quicker recovery of gastrointestinal motility with shorter time to first flatus and oral intake, and shorter hospital stay [34]. A randomized prospective trial comparing laparoscopic assisted with open subtotal gastrectomy reported that LG had a significantly lower blood loss (229 ± 144 ml versus 391 ± 136 ml; P < 0.001), shorter time to resumption of oral intake (5.1 ± 0.5 days versus 7.4 ± 2 days; P < 0.001), and earlier discharge from hospital (10.3 ± 3.6 days versus 14.5 ± 4.6 days; P < 0.001) [35] (Table 9.2).
Table 9.2
Trials comparing laparoscopic subtotal gastrectomy with open subtotal gastrectomy
Author, year | N | Average # LNs retrieved | Mortality | Morbidity | 5-year OS | 5-year DFS | Comments |
|---|---|---|---|---|---|---|---|
Fujii, 2003 [36] | 20 | N/A | OG = 0 % | OG = 20 % | N/A | N/A | LG had |
LG = 0 % | LG = 20 % | Longer operative time | |||||
P = NS | P = NS | ||||||
Hayashi, 2005 [37] | 28 | OG = 27 ± 10 | N/A | N/A | N/A | N/A | LG had |
LG = 28 ± 14 | |||||||
Longer operative time | |||||||
P = .85 | |||||||
Earlier oral intake | |||||||
Shorter LOS | |||||||
Lee, 2005 [38] | 47 | OG = 38.1 ± 15.9 | N/A | N/A | N/A | N/A | LG had |
LG = 31.8 ± 13.5 | |||||||
N = 0.406 | |||||||
Longer operative time | |||||||
Lower pulmonary complications | |||||||
Huscher, 2005 [35] | 59 | OG = 33.4 ± 17.4 LG = 30.0 ± 14.9 | OG = 6.7 % | OG = 27.6 % | OG = 55.7 % | OG = 58.9 % | LG had |
LG = 54.8 % | |||||||
LG = 57.3 % | Lower EBL | ||||||
LG = 3.3 % | LG = 26.7 % | ||||||
Earlier oral intake | |||||||
P = NS | P = NS | ||||||
P = NS | |||||||
Shorter LOS | |||||||
P = NS | |||||||
P = NS | |||||||
Kim, 2008 [39] | 164 | OG = 45.1 ± 13.8 | N/A | OG = 5 % | N/A | N/A | LG had |
LG = 0 % | Longer operative time | ||||||
N/A | |||||||
LG = 39.0 ± 11.9 | |||||||
Lower EBL | |||||||
Shorter LOS | |||||||
Better QOL | |||||||
P < 0.05 | |||||||
Kim, 2010 [40] | 342 | OG = 71 % had D2 | OG = 0 % | OG = 14.7 % | N/A | N/A | No differences in morbidity or mortality between OG and LG |
LG = 1.1 % | LG = 10/5 % | ||||||
P = 0.497 | P = 0.137 | ||||||
LG = 67 % had D2 | |||||||
P = 0.48 |
Laparoscopic subtotal gastrectomy appears to offer similar oncologic outcomes as open subtotal gastrectomy as reported in multiple retrospective studies [34, 41–44] as well as in six randomized controlled trials [35–40] (Table 9.2). As an aside, there are no clinical trials comparing laparoscopic total gastrectomy with open total gastrectomy. Unfortunately, most of these clinical trials had small number of patients; the highly quoted study by Huscher et al. reported only 59 patients [35]. To address the concern of insufficient power, the Korean Laparoscopic Gastrointestinal Surgery Study (KLASS) Group is conducting a large, multicenter phase III trial of 1,415 patients from 12 institutions to compare overall survival, disease survival, morbidity, mortality, quality of life, inflammatory and immune responses, and cost-effectiveness between laparoscopic distal gastrectomy and open distal gastrectomy on early stage gastric cancer (cT1N0M0-cT2aN0M0) (Clinical Trials.gov ID: NCT00452751) [40]. A separate trial comparing laparoscopic surgery with open surgery in advanced gastric cancer is also underway (KLASS 02). Results of these studies will not be available until the distant future.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree