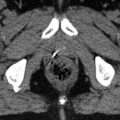
Figs. 9.1 and 9.2
LN drainage: Compartment 1 (DI resection): 1. right cardial nodes; 2. left cardial nodes; 3. nodes along the lesser curvature; 4. nodes along the greater curvature; 5. suprapyloric nodes; 6. infrapyloric nodes. Compartment 2 (D2 resection): 7. nodes along the left gastric artery; 8. nodes along the common hepatic artery; 9. nodes around the coeliac axis; 10. nodes at the splenic hilus; 11. nodes along the splenic artery; 12. nodes in the hepatoduodenale ligament; 13. nodes at the posterior aspect of the pancreas head; 14. nodes at the root of the mesenterium; 15. nodes in the mesocolon of the transverse colon; 16. paraaortic nodes. (Hartgrink and van de Velde 2005)
9.3 Pattern of Failure
Awareness of pattern of failure after surgery, as well as knowledge of the relevant anatomy and organs at risk (OAR), must be the basis for target volume definition. In clinical, reoperative, and pathologic studies, mainly four different sites of failure after surgery have been described: local recurrence, LN failure, peritoneal metastasis, or distant metastasis. Results of pattern of failure based on reoperation series in charge of the University of Minnesota and autopsy studies are summarized in Table 9.1 (Gunderson and Sosin 1982). These findings imply that mainly local recurrence of the tumor bed, in the area of anastomotic junction, and local-regional LN failure appeared after surgery. Some autopsy studies even described local-regional failure rates up to 93 % (Horn 1955). These results demonstrate the potential benefit of local-regional radiotherapy.
University of Minnesota reoperation series (n = 107) | Autopsy series | |
|---|---|---|
Pattern of relapse | % | % |
Gastric bed | 55 | 52–68 |
Anastomosis | 27 | 54–60 |
Abdominal wound | 5 | – |
Peritoneal seeding | 42 | 30–43 |
Lymph nodes | 43 | 52 |
Local-regional as any site | 69 | 80–93 |
Local-regional only | 23 | – |
Lymph nodes only | 7 | – |
Lymph nodes only in nodal dissection sites | 3 | – |
Distant metastases | 36 | 50 |
Pattern of lymphatic spread differs according to the localization of the primary tumor. On the one hand, tumors localized in the fundus tend to spread to splenic nodes (12–42 %), but less commonly to subpyloric lymph nodes. On the other hand, subpyloric nodes (~50 %) constitute the primary site of lymphatic spread of antral tumors, and splenic nodes are rather rarely involved (<10 %) (Smalley et al. 2002).
However, peritoneal seeding is relatively frequent in gastric cancer and has been described in the literature in up to 43 % of postgastrectomy patients (Gunderson and Sosin 1982).
Distant metastasis can be found most commonly in the liver, in approximately 30 % of cases and 10 % in the lung. Yet, with effective locoregional therapy, these secondary failures could be averted (Fig. 9.3).
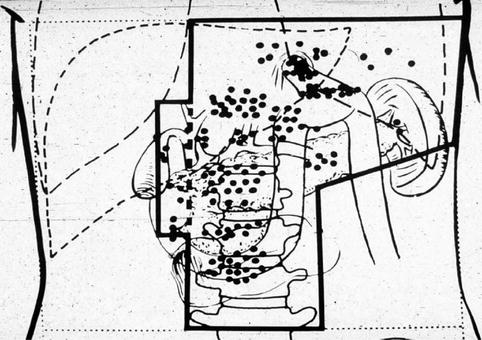

Fig. 9.3
Gunderson cartoon of hypothetical radiotherapy field based on the University of Minnesota reoperation series (Gunderson and Sosin 1982)
9.4 Immobilization and Simulation
Patients are usually simulated in supine position with arms raised above the head. For enhancing reproducibility, a variety of immobilization systems, for example, wing boards and vacuum cushions, are available. Legs are placed on knee fixations and patients should be advised to fast for 3 h before simulation.
CT-based radiotherapy planning for gastric cancers is obligatory. CT images should be acquired from above the diaphragm to below the kidneys at the level of vertebrae L4/L5. Application of intravenous contrast is useful to identify the tumor and lymph nodes, but administration of oral contrast medium to visualize gastric distension is only recommended for diagnostic CT, not for planning CT. Surgical clips may be used for matching, but the radiation oncologist must be aware that the anatomy changes after surgery and that clips may not represent the resection margin, but rather vessel clips. Optimally, additional imaging like MRI or PET can be used for treatment planning and available CT slices should not exceed a thickness of 3 mm.
9.5 Target Volume Definition
Accurate target volume definition is based on the surgical and pathology reports and the combination of imaging, including CT, endoscopy, and endoscopic ultrasound (EUS) as compulsory and PET as recommended modalities.
9.5.1 Neoadjuvant and Definitive Radiation
For neoadjuvant and definitive radiotherapy treatment, the gross tumor volume (GTV) typically encloses the primary tumor (GTV-tumor) and all involved lymph nodes (GTV-nodal). The clinical target volume (CTV) includes microscopic disease with the primary tumor and their regional lymphatics. In the past, the whole stomach was irradiated, but to minimize toxicity, the target volume now depends on the location of the primary tumor (PT) within the stomach and whether imaging has detected nodal metastasis:
For PT located in the fundus, the CTV includes the stomach with exclusion of pylorus, antrum, and pancreaticoduodenal lymph node region.
For PT located in the corpus, the CTV includes the whole stomach with the regional lymph nodes.
For PT located in the antrum, the CTV includes the stomach without fundus/cardia and splenic lymph node region.
Regardless of the location, a minimum margin of 5 cm in all directions to the GTV must be added. The internal target volume (ITV), which takes the target motion into account, should then be designed. Yet, 4-dimensional computed tomography (4D-CT) planning is not widely available. Therefore, the EORTC-ROG recommends to expand the CTV 1.5 cm distally, 1 cm radially, and 1 cm proximally to create the ITV (Matzinger et al. 2009). At least, a 5 mm 3-D margin is added to the ITV to construct the PTV.
9.5.2 Adjuvant Radiation
Optimally, the GTV has been completely resected at surgery, so the CTV encompasses the tumor bed with a 2 cm range proximally and distally of resection margins, as well as the anastomosis area and the perigastric nodes on the greater and lesser curvature.
Before encompassing the target volume, the radiation oncologist should study surgery and pathology reports carefully. Preoperative images of the GTV are useful to reconstruct the initial tumor localization on the present planning CT.
Adjuvant radiation of the tumor bed in widely resected T1 to T2a tumors is optional, but highly recommended for tumor stages T2b to T4.
In addition, if the margin is less than 5 cm or if lymph nodes are involved, the residual stomach should be included in the CTV. In case of a proximal gastric tumor, 3–5 cm of the distal esophagus should be included in the CTV.
LN irradiation should be performed in any case, but the radiation volume is depending on the site of the primary tumor. If the primary tumor is localized in the upper third of the stomach, infrapyloric and gastroduodenal lymph nodes may be omitted. In the same manner, for small gastric tumors of the lower third, splenic LN may be excluded from CTV.
9.5.3 Organs at Risk (OAR)
To reduce toxicity, the whole volume of all OAR is contoured on the planning CT.
The maximal spinal cord dose must be kept below 45 Gy, which is usually not a problem; 70 % of the volume of each kidney should not receive >20 Gy. Assuming that major parts of the kidney receive more than 20 Gy, the glomerular filtration rate of both kidneys should be measured before RT, to ensure that a sufficient renal function exists. At least 30 % of the liver should not receive beyond 30 Gy (V30 <30 %). Moreover, 30 % of the heart must not receive over 40 Gy and half of the heart must be kept under a total dose of 25 Gy. Attempts should also be made to minimize dose to the lungs, so less than 20 % should receive >20 Gy (V20 <20 %) (Table 9.2).
Table 9.2




Overview of radiation margins and tolerance of OAR
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree