Fig. 11.1
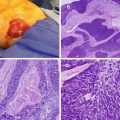
(a) The gallbladder (green) is contiguous with segments 4b and 5 of the liver. Segment 1 (caudate lobe) is not shown. (b) Couinaud classification of the liver divides the liver into eight segments. (c) Illustration of the close relationship of the gallbladder to surrounding organs: the liver, duodenum, and transverse colon. Moreover, the cystic duct and neck of the gallbladder are in close proximity to the structures in the hepatoduodenal ligament: the common bile duct (green), portal vein (blue), and hepatic artery (red). (d) Layers of the gallbladder wall: the portion facing the liver is indicated in red, and the portion facing the peritoneal cavity is indicated in blue (Courtesy of Quyen D. Chu, MD, MBA, FACS)
The intraperitoneal portion of the gallbladder is covered with (visceral) peritoneum or serosa (Fig. 11.1d). If the cancer extends beyond the serosa of the gallbladder, it may involve surrounding organs such as the stomach, duodenum, pancreas, or transverse colon (Fig. 11.1c). The part of the gallbladder facing the liver has no peritoneal covering: only a layer of perimuscular connective tissue called the cystic plate separates the muscularis of the gallbladder from the liver parenchyma (Fig. 11.1d). A simple cholecystectomy involves dissection between the muscularis of the gallbladder and the cystic plate. Consequently, if GBC is discovered at pathologic review after a simple cholecystectomy, the resection margin is likely involved, unless the tumor did not invade the muscularis and is limited to the lamina propria (T1a).
GBC typically arises in the mucosa of the gallbladder, with adenocarcinoma or its variants (adenosquamous, squamous) found in 98 % of all patients. Rare histologies of the gallbladder include neuroendocrine tumors, sarcomas, or metastatic diseases such as melanoma. The most common infiltrative subtype invades the entire gallbladder in the subserosal plane, followed by invasion of the liver and the porta hepatis. The nodular subtype forms a more circumscribed lesion; the papillary type forms polypoid lesions and is less invasive.
Dye studies have demonstrated the route of lymph flow from the gallbladder first to the cystic duct node and the nodes around the bile duct, then to nodes around the hepatic vessels and posterior to the pancreas, and finally to the aortocaval nodes near the left renal vein (Fig. 11.2) [8]. In some patients, additional lymphatics are found connecting regional lymph nodes directly to aortocaval nodes. Positive lymph nodes beyond the hepatoduodenal ligament (i.e., periaortic, pericaval, superior mesenteric artery, and/or celiac artery lymph nodes) are considered stage IV disease since the seventh edition of the American Joint Committee on Cancer (AJCC) TNM classification for GBC [9].
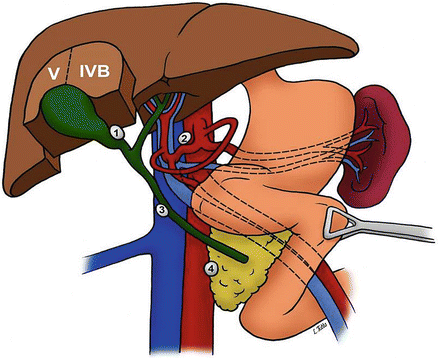

Fig. 11.2
Regional lymphadenectomy for gallbladder cancer includes lymph nodes in porta hepatis, hepatoduodenal and gastrohepatic ligament, and retroduodenal regions
Lymph Node Stations for Gallbladder Cancer
1: Cystic duct lymph node
2: Common hepatic artery lymph node
3: Portocaval lymph nodes
4: Common bile duct lymph nodes (Courtesy of Quyen D. Chu, MD, MBA, FACS)
Although lymphatic spread is important for staging, spread to distant sites occurs mainly through hematogenous dissemination, either directly or associated with invasion into the liver parenchyma [10]. When indocyanine green is injected in the cystic artery of GBC patients, the dye extends up to 4 cm into the parenchyma of segments 4b and 5 of the liver [11]. In an immunohistochemical study of liver resections for GBC, intrahepatic portal vein invasion was detected in more than half of the patients, up to 12 mm beyond the border of direct invasion. Metastatic nodules were found in 26 % of GBC patients, on average 16 mm beyond the border of direct invasion [12].
Table 11.1 presents the AJCC seventh edition guideline for staging of GBC, which is based on the anatomical considerations described above, as well as prognostic research [9]. Figure 11.3a presents overall survival of more than 10,000 patients with GBC diagnosed in the years 1989–1996 [9]. These data are from the National Cancer Data Base. Figure 11.3b presents overall survival of patients with GBC who had surgery, stratified by T stage and N stage [13]. These data are from the Surveillance, Epidemiology, and End Results (SEER) program, representing 26 % of the US population for the period 1991–2005. Patients with metastatic disease and stage T4 at the time of surgery were excluded [13].
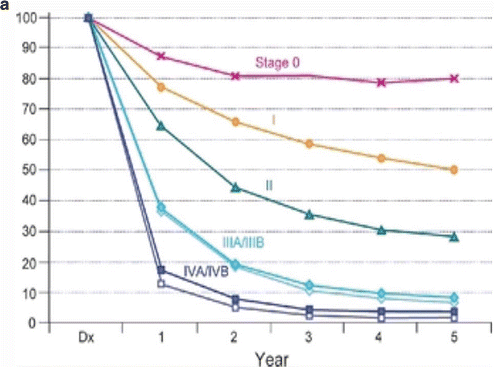
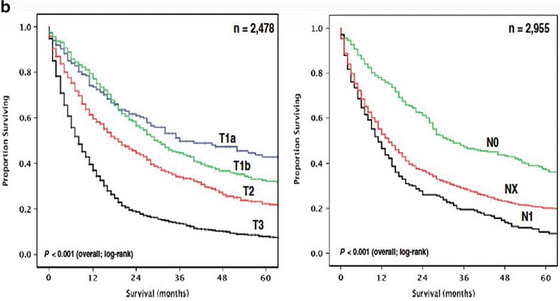
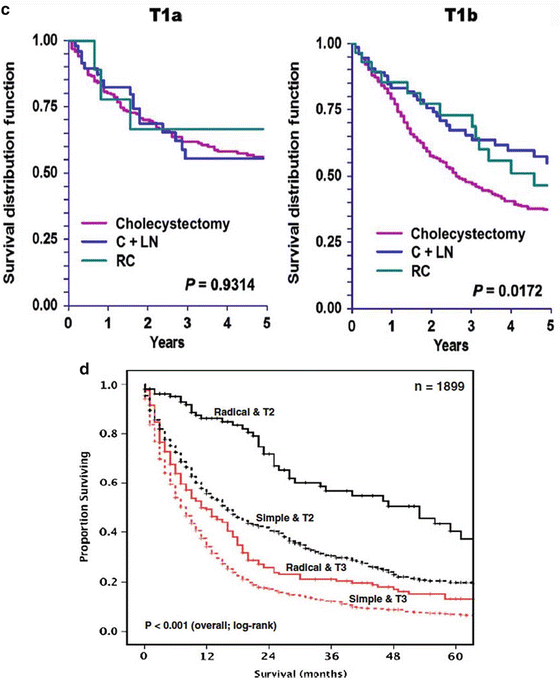
Table 11.1
American Joint Committee on Cancer (AJCC) TNM staging for gallbladder cancer (seventh edition)
Primary tumor (T) | |
|---|---|
TX | Primary tumor cannot be assessed |
T0 | No evidence of primary tumor |
Tis | Carcinoma in situ |
T1a | Tumor invades lamina propria |
T1b | Tumor invades muscular layer |
T2 | Tumor invades perimuscular connective tissue; no extension beyond serosa or into liver |
T3 | Tumor perforates the serosa (visceral peritoneum) and/or directly invades the liver and/or one extrahepatic organ or structure* |
T4 | Tumor invades main portal vein or hepatic artery or invades two or more extrahepatic organs or structures* |
*Extrahepatic organs or structures include the stomach, duodenum, colon, pancreas, omentum, and extrahepatic bile ducts | |
Regional lymph nodes (N) | |
|---|---|
NX | Regional lymph nodes cannot be assessed |
N0 | No regional lymph node metastasis |
N1 | Metastases to nodes along the cystic duct, common bile duct, hepatic artery, and/or portal vein |
N2 | Metastases to the periaortic, pericaval, superior mesenteric artery, and/or celiac artery lymph nodes |
Distant metastasis (M) | |
|---|---|
M0 | No distant metastasis |
M1 | Distant metastasis |
Anatomic stage/prognostic groups | |||
|---|---|---|---|
Group | T | N | M |
Stage 0 | Tis | N0 | M0 |
Stage I | T1 | N0 | M0 |
Stage II | T2 | N0 | M0 |
Stage IIIA | T3 | N0 | M0 |
Stage IIIB | T1-3 | N1 | M0 |
Stage IVA | T4 | N0-1 | M0 |
Stage IVB | Any T | N2 | M0 |
Any T | Any N | M1 | |



Fig. 11.3
(a) Overall survival of more than 10,000 patients with GBC diagnosed in the years 1989–1996. Data from the National Cancer Data Base [9]. (b) Overall survival for patients with gallbladder cancer who had surgery, stratified by T stage (p < 0.001) and N stage (P < 0.001). Data from the SEER database, representing 26 % of the US population, for the period 1991–2005. Patients with metastatic disease and stage T4 at the time of surgery were excluded. (c) Overall survival in patients with stage T1a (n = 300) and T1b (n = 536) GBC, stratified by type of surgery. C + LN, cholecystectomy with any lymph node dissection; RC, radical (i.e., extended) cholecystectomy including liver resection and regional lymph node dissection. Data from the SEER database, representing 26 % of the US population, for the period 1988–2008. (d) Overall survival in patients with stage T2 and T3 GBCs, further stratified by type of surgery. Radical, radical (i.e., extended) cholecystectomy including liver resection; simple, simple cholecystectomy [13]. Data from the SEER database, representing 26 % of the US population, for the period 1991–2005 ((a) Reprinted from Edge [9]. With permission from Springer Verlag. (b) Reprinted from Mayo et al. [13]. With permission from Springer Verlag. (c) Reprinted from Hari et al. [44]. With permission from John Wiley & Sons, Inc. (d) Reprinted from Mayo et al. [13]. With permission from Springer Verlag)
Gallbladder Polyp on Imaging
Polypoid lesions and focal wall thickening of the gallbladder are found on ultrasound (US) in 3–7 % of healthy adults [14–16]. Their incidence has increased with more frequent use and improved resolution of US. Only rarely do they cause symptoms by obstructing the gallbladder outlet. Although most of these lesions are benign, some are premalignant (adenomatous polyps), and rarely GBC is found. The risk of malignant transformation of gallbladder polyps, while possible, is extremely low for small lesions (<1 cm) and appears to be lower than previously reported for larger lesions (>1 cm). Additionally, it should be recognized that small polypoid lesions are very often nonneoplastic.
Ultrasound cannot reliably distinguish premalignant polyps from pseudopolyps such as cholesterol polyps. The size of the polyp is an important predictor of malignancy. Based on a study in 1982, surgical resection has been recommended for polyps of at least 10 mm, because GBC was found only in polyps larger than 12 mm [17]. More recent studies confirmed that malignancy is extremely rare, if found at all, in polyps smaller than 10 mm, except in patients with primary sclerosing cholangitis (PSC) [18–20]. Of all polyps found on US, 7–29 % are larger than 10 mm. Risk factors for malignancy of polyps other than size and PSC are age above 50 years, Indian ethnic background, a sessile polyp, a single polyp, and the presence of gallstones [14, 19]. In patients with polyps smaller than 5 mm on US, no polyp or mass is found on pathologic examination in up to 83 %; for polyps larger than 20 mm, cancer may be present in up to 59 % [19]. Cholecystectomy for polyps of at least 10 mm remains a valid guideline based on recent series. In addition, cholecystectomy for polyps of more than 5 mm in patients with PSC appears justified, given the much higher risk of malignancy in that setting.
The resolution of conventional US is insufficient to distinguish the layers of the gallbladder wall (Fig. 11.1d). Therefore, US has a poor diagnostic accuracy for detecting a polyp that harbors GBC invading into or just beyond the lamina propria. Although CT can detect invasion of the liver parenchyma (T3) and distant metastases, its diagnostic accuracy for depth of invasion is also poor. Diagnostic accuracy for depth of invasion appears better for high-resolution US (63 %) and endoscopic ultrasound (56 %) [21]. In patients with a high likelihood of GBC (e.g., polyps larger than 15 mm), but no invasion on conventional US, a preoperative high-resolution US or endoscopic US may alter surgical management.
A simple cholecystectomy is sufficient for polyps and early (i.e., T1a) GBC (see below). Cholecystectomy can be performed open or laparoscopically. Bile spillage should be avoided because, if GBC cells are present in the bile, they can cause peritoneal or port-site metastases. The incidence of bile spillage during laparoscopic cholecystectomy for incidental GBC was about 20 % in a Japanese series of 498 patients and was associated with a higher recurrence rate of 27 % versus 14 % if no spillage occurred [22]. Other series showed that port-site or incisional recurrences occurred at least twice as often in laparoscopic versus open cholecystectomy for GBC [23, 24]. Even for in situ carcinoma, peritoneal dissemination has been described after gallbladder perforation [25]. A low threshold for conversion to open cholecystectomy is therefore recommended. A bag should be used for laparoscopic removal of the gallbladder. For patients with an increased risk of malignancy (e.g., polyp larger than 15 mm), open cholecystectomy should be considered because of the increased risk of bile spillage and peritoneal dissemination with laparoscopic resection. A simple cholecystectomy may not result in clear margins if GBC with invasion beyond the lamina propria is found. Frozen section of the gallbladder could be obtained to rule out GBC, if expertise is available for immediate liver resection (segments 4b and 5) and lymphadenectomy, but there is a high risk of sampling error in this setting. If the GBC is limited to the lamina propria (i.e., stage T1a), additional resection is not required [3].
Regarding polyps smaller than 10 mm that are not resected, the question arises whether follow-up is necessary. A study from 1962 found no GBC during a 15-year follow-up of patients with gallbladder polyps [26]. In a recent study, a follow-up US was available for 149 patients, 2–12 years after the initial US: increase in size was noted in only 1 polyp (from 3 to 5 mm, not clinically relevant), and two thirds of these small polyps were undetected at follow-up [27]. In another recent series, growth was seen in 8 out of 143 patients during follow-up, but no cancer developed [18]. On the other hand, in a small series of patients with gallbladder polyps, rapid growth was found on repeat US in the months before surgery in five patients who eventually had GBC demonstrated [28]. Because of the conflicting data, follow-up at 6- to 12-month intervals for 2 years is generally recommended.
Several other gallbladder wall lesions can be found on imaging or during surgery. Calcification of the gallbladder wall, or porcelain gallbladder, appears to increase the risk of malignant transformation. However, the risk appears to be lower than was suggested based on older studies and also seems to be related to the nature of the calcifications (i.e., diffuse versus discontiguous or selective). In a number of studies, patients with diffuse calcification of the gallbladder had no GBC identified on histopathologic analysis [29–31]. However, one study of more than 25,000 resected gallbladders found GBC in 2 out of 27 patients with selective mucosal calcification of the gallbladder wall [30]. Cholecystectomy for patients with selective mucosal calcification is therefore recommended.
Adenomyomatosis of the gallbladder is characterized by focal thickening of the gallbladder wall with cystic-appearing spaces (Rokitansky-Aschoff sinuses) that are identified with high accuracy on US. Because these lesions are invariably benign and asymptomatic, they need no surgical management or follow-up [32].
Xanthogranulomatous cholecystitis is an uncommon inflammatory disease of the gallbladder with extensive fibrosis that can present with wall thickening, mass formation, and infiltration of the liver and other adjacent organs [33]. Typical findings on imaging include diffuse gallbladder wall thickening, hypo-attenuating intramural nodules, continuous mucosal line enhancement, and the presence of gallstones [34]. However, accuracy of these findings is often insufficient to rule out GBC, and xanthogranulomatous cholecystitis is typically diagnosed at pathologic review after extended cholecystectomy (i.e., segment 4b and 5 resection en bloc with gallbladder). In a series from India comprising 198 patients resected for presumed GBC, 16 % was found to have xanthogranulomatous cholecystitis [35].
Incidental Gallbladder Cancer at Pathologic Review
Incidental GBC is found on pathologic review of about 1 % of laparoscopic cholecystectomy specimens [36–38]. These patients comprise about two thirds of all patients with potentially curable GBC. In most of these cases, the gallbladder is resected for presumed symptomatic cholelithiasis, and GBC was not suspected on preoperative imaging or during surgery. Many of these patients benefit from reoperation and definitive resection. Re-resection may be beneficial if residual cancer is limited to the liver bed, cystic stump, common bile duct, or lymph nodes in the absence of distant metastasis. A large Western study showed that 14 % of these incidental GBC patients had disseminated disease on re-exploration, and 73 % of re-resected patients had residual disease on final pathology [39, 40].
The probability of both distant metastases and local residual cancer increases with the depth of invasion (i.e., T stage). In a Japanese nationwide survey of 498 patients with incidentally found GBC, 34 % had stage T1a, 14 % T1b, 41 % T2, 8 % T3, and 2 % T4 [22]. Table 11.2 presents the probability of residual disease in the liver or regional lymph nodes stratified by T stage, found in re-resected patients with incidental GBC [41]. In another large Western series, the median survival time was 15 months if residual disease was found (73 % of patients), compared with 72 months if no residual disease was found [40]. The National Comprehensive Cancer Network (NCCN) guideline recommends re-resection for non-metastatic patients with T1b to T3 GBC [3]. Appropriate re-resection should include, at a minimum, resection of the liver segments contiguous with the gallbladder (segments 4b and 5) and regional lymphadenectomy, with selective bile duct resection (Fig. 11.2). The surgical procedures are described in more detail below.
Table 11.2
Residual disease found at re-resection for incidental GBC
T stage | Number of patients | Percentage of all stages (%) | Residual disease –anya (%) | Residual disease – liver (%) | Residual disease – nodes (%) |
|---|---|---|---|---|---|
T1 | 8 | 8 | 38 | 0 | 13 |
T2 | 67 | 68 | 57 | 10 | 31 |
T3 | 22 | 22 | 77 | 36 | 46 |
All stages | 97 | 100 | 59 | 15 | 33 |
Many studies have evaluated the benefit of a re-resection in patients with pT1a and pT1b GBC. A systematic review of T1 GBC identified 29 retrospective studies representing 1,266 patients [42]. T1a GBC was found in 56 % of all T1 GBC patients, of whom 16 % underwent re-resection. T1b was found in 44 %, of whom 33 % underwent re-resection. Patients with T1a GBC had lymph node metastases in 2 % and patients with T1b in 11 %. Eight patients (1 %) with T1a GBC died of recurrence. Fifty-two patients (9 %) of all patients with T1b died of recurrence: 13 % recurred after simple cholecystectomy alone and 3 % recurred after definitive re-resection. In a German prospective registry, 23 of 72 patients with T1b GBC underwent a re-resection [43], and this was associated with a 3-fold lower recurrence rate and a 5-year overall survival of 79 versus 42 months for simple cholecystectomy only (P = 0.03). In a study of more than 1,000 T1 GBC patients in the Surveillance, Epidemiology, and End Results (SEER) database, 80 % of both T1a and T1b patients underwent only a simple cholecystectomy [44]. For T1b patients, survival was better when combined with a liver resection and/or lymphadenectomy; for T1a patients, survival was similar with more extensive surgery (Fig. 11.3c). The NCCN guideline therefore recommends re-resection after an incidentally found T1b GBC [3]. For T1a GBC, the recurrence rate of a simple cholecystectomy alone is similar to the postoperative mortality rate for re-resection (approximately 1.5 %) [42]. Re-resection after an incidentally found T1a GBC is, therefore, not recommended.
Patients with T2 and T3 GBC may appear more likely to benefit from re-resection, because they are more likely to harbor residual disease than T1 GBC. However, they are also more likely to harbor occult distant metastatic disease, in which case re-resection is of no benefit. Several studies have suggested a benefit of re-resection for both T2 and T3 GBCs, using data from the SEER cancer registry [13, 45, 46]. In 781 patients with T2 GBC, the median survival time and the 5-year survival rate were 53 months and 37 %, respectively, after re-resection compared with 16 months and 21 % after cholecystectomy alone (Fig. 11.3d). In 1,118 patients with T3 GBC, the median survival and the 5-year survival rate were 11 months and 13 %, respectively, after re-resection compared with 8 months and 8 % with cholecystectomy alone (Fig. 11.3d) [13]. The benefit of re-resection persisted in node-positive patients with T1b or T2 tumors, but a benefit was not detected in node-positive patients with T3 tumors. In a German registry of 200 patients with incidental T2 GBC, 85 patients underwent re-resection, resulting in a 5-year survival of 55 % versus 35 % for patients subjected to a simple cholecystectomy [47]. In the same registry, of the 85 patients with T3 GBC, 32 underwent re-resection, but this did not result in an obvious improvement in the 5-year survival, which was only 18 %. Single institution series are smaller than these registries, but also found better survival after re-resection, especially for T2 tumors [48, 49]. The survival benefit found in these nonrandomized studies could be at least partly due to selection bias. In other words, there may well have been a good reason to exclude certain patients from re-resection that is not reflected in or is impossible to assess in retrospective analyses. Re-resection after incidentally found T2 and T3 GBCs is recommended, although the benefit is probably small for T3 GBC, and patients with node-positive T3 GBC may not benefit at all from re-resection. Unfortunately, nodal status is typically unknown after simple cholecystectomy and may not be known with certainty until the final histological analysis is complete. Table 11.3 summarizes the management of GBC patients based on T stage.
Table 11.3
Management of GBC patients based on T stage
T stage | Recommendation |
|---|---|
T1a | Simple cholecystectomy |
T1b–T2 | Cholecystectomy with en bloc liver resection of segments 4b and 5 (anatomical or wedge) with lymph node dissection of the hepatoduodenal ligament |
T3 | As for T1b–T2 but GBC in the gallbladder neck or the cystic duct may require right hepatectomy extended to segment 4b and/or bile duct resection with hepaticojejunostomy to obtain clear margins. To ensure negative margins, any adherent organ or structurea should also be resected |
T4 | As for T3, resection of two or more adherent organs or structuresa can be considered. Palliative care if involvement of main portal vein or proper hepatic artery. Most patients in this category are not candidates for resection or will not benefit from resection even if feasible technically |
Before embarking on re-resection, distant metastases should be ruled out using abdominal CT or MRI and imaging of the chest. Positron-emission tomography (PET) may be of some benefit in avoiding futile surgery by detecting metastatic disease not found on cross-sectional imaging [50]. In one study, PET found distant metastasis in 3 out of 23 patients with incidental GBC [51]. As part of the work-up, it is important to review the initial imaging before cholecystectomy, the operative note, and the pathology report. These may inform on the precise location of the tumor within the gallbladder (on the peritoneal or liver side; near the fundus or near the cystic duct), inadvertent perforation of the gallbladder, and margin status at the cystic duct. If the cystic duct margin is positive for invasive cancer or high-grade dysplasia, bile duct resection is recommended to obtain clear margins. However, this information is often not included in routine histologic assessment for cholecystectomy performed for presumed benign disease. In a Western series, 8 out of 19 patients with a positive cystic duct margin had residual disease in the common bile duct [41]. Alternatively, re-resection of the cystic duct stump with frozen section could be considered [52].
Staging laparoscopy may prevent an unnecessary exploratory laparotomy if disseminated disease is found. The incidence of metastatic disease at re-exploration for GBC is 14–24 % [39, 41]. Of 46 patients undergoing staging laparoscopy in a Western series, 10 (22 %) had metastatic disease that was identified laparoscopically in only two patients, whereas in the other eight it was found at laparotomy [39]. Peritoneal metastasis is more likely in patients with poorly differentiated or T3 tumors, or after perforation of the gallbladder at cholecystectomy. The yield of staging laparoscopy would appear to be greater in such high-risk patients, and it is reasonable to use it routinely in these settings.
Incidental Gallbladder Cancer Found at Surgery
GBC is sometimes suspected during surgery, typically during laparoscopic cholecystectomy for cholelithiasis. If peritoneal or hepatic lesions are found and frozen section demonstrates GBC, the patient has metastatic disease and will not benefit from resection. If the diagnosis of GBC is suspected based on macroscopic assessment of the gallbladder and no expertise in GBC is available, it is probably best to not perform any resection and refer the patient to a specialized hepatobiliary center where the disease can be staged fully and the tumor resected in a single definitive procedure observing oncologic principles. Studies that report no difference in survival between patients undergoing two resections or a single definitive resection likely suffer from selection bias, since patients who develop disseminated disease prior to the second procedure are excluded [48, 53].
GBC may also be suspected after cholecystectomy by macroscopic assessment of the gallbladder mucosa. If the mucosa appears abnormal, then the gallbladder can be sent for frozen section histology, and definitive resection may be undertaken at that time.
Mass Found on Imaging, Suspicious for GBC
GBC is typically asymptomatic until advanced stages, when involvement of the liver and other surrounding structures occurs. At this stage, patients may present with constant right upper quadrant pain, weight loss, and nausea with vomiting. About 40 % of patients are jaundiced at presentation, an ominous finding [54]. At an advanced stage, US often demonstrates a heterogeneous mass in the gallbladder. Sometimes a diffuse thickening of the gallbladder wall is seen that can be difficult to distinguish from cholecystitis. Complete cross-sectional imaging, usually in the form of CT of the chest, abdomen, and pelvis, is recommended as the next step. PET scan may be justified in selected patients, to assess suspicious findings at distant sites, but is probably not helpful as a routine study [51].
The NCCN guideline has the same recommendations for these patients, as for patients in whom GBC was incidentally found [3]. In summary, most non-metastatic patients are expected to benefit from surgical resection. Exceptions are patients with positive lymph nodes beyond the hepatoduodenal ligament (i.e., the periaortic, pericaval, superior mesenteric artery, and/or celiac artery lymph nodes), which is considered stage IV disease according to the seventh edition of the AJCC TNM classification. Moreover, patients with T4 tumors are unlikely to benefit from surgery even if their disease is amenable to resection. T4 tumors are locally advanced and include those that invade or encase the main portal vein and the hepatic artery or involve at least two extrahepatic organs or structures (e.g., the duodenum, pancreas, and transverse colon). If a patient has substantial comorbidities, a trade-off should be made between the expected postoperative morbidity and mortality and the long-term oncologic benefits of surgery.
The prognosis of symptomatic (non-incidental) GBC patients remains poor, even after liver segment 4b and 5 resection with lymphadenectomy of the hepatoduodenal ligament. In a Western series including 54 patients with non-incidental GBC, 48 patients underwent surgical exploration of which only 11 underwent a liver resection. The median survival was only 8 months [53].
Controversies in Surgical Management
In the absence of data from prospective, controlled trials, many issues pertaining to the treatment of patients with GBC remain unresolved. This section highlights three common controversies: the extent of liver resection, indications for extrahepatic bile duct resection, and the benefit of laparoscopic port-site resection.
The first controversy concerns whether GBC patients should undergo a liver wedge resection of 1–5 cm, an anatomic resection of segments 4b and 5, or an extended right hepatectomy. In 1954, Glenn and Hays first proposed a liver resection for GBC: the gallbladder was resected en bloc with a 1 cm wedge of liver parenchyma [55]. Since then, wider liver resections have been recommended to obtain clear margins, eliminate micrometastases in the liver, and ultimately avoid recurrence in the liver [56]. The results of histological studies support an anatomic resection of segments 4b and 5 (see section on anatomy) [12, 57]. However, in a Japanese multicenter series of 485 R0 T2/3 patients, no difference in survival or local recurrence was found between wedge resection, anatomical segmental resection, or extended right hepatectomy [58]. Metastases in the liver beyond segments 4b and 5 represent stage IV disease, and survival beyond 1 year is rare; extended hepatectomy appears futile for this indication. Moreover, the postoperative mortality of extended liver resections was between 9 and 18 % in several series [59–61]. Postoperative mortality occurred mainly in the setting of liver failure due to the extensive resection in combination with obstructive jaundice in 45–100 % of these patients. Therefore, most surgeons perform a wedge resection of about 2 cm liver parenchyma (en bloc with the gallbladder, if still in situ) or an anatomic liver resection of segments 4b and 5. An extended right hepatectomy (extended to segment 4b, with or without 4a and 1) could be considered in medically fit patients with GBC arising in the gallbladder neck, Hartmann’s pouch, or the cystic duct. These tumors are close to the right hepatic pedicle at an early stage, and a conventional segment 4b and 5 liver resection is likely to result in a positive margin (see section on anatomy) [49].
The second controversy concerns resection of the extrahepatic bile duct (EBD) for patients with GBC. Involvement of the EBD often results in jaundice. EBD resection for jaundiced GBC patients was traditionally performed as a matter of routine, although enthusiasm has waned with the publication of poor outcomes [62]; however, this practice is still recommended by some Asian surgeons [60, 62]. In a Western series of 82 GBC patients who presented with jaundice, 55 were explored of whom 6 were resected (including EBD resection), of whom 4 had an R0 resection [62]. All six resected patients died or recurred within 6 months. The median survival of all jaundiced GBC patients was 6 months and all patients died before 28 months. Of the 82 jaundiced patients, only three patients had node-negative disease. Because of these poor outcomes, the NCCN guideline recommends to consider resection only in node-negative jaundiced GBC patients, at an experienced center; however, definitive determination of nodal status prior to operation can be difficult [3]. Jaundice may be an early event in patients with GBC arising from the gallbladder neck, Hartmann’s pouch, or the cystic duct. In theory, this small subgroup may benefit from resection. These patients have likely involvement of the right portal pedicle and require a combined extended right hepatectomy, EBD resection, and regional lymphadenectomy to obtain clear margins.
In the absence of jaundice, EBD resection in GBC patients is recommended to obtain clear margins for a positive cystic duct margin after a previous cholecystectomy. In one study, 8 of 19 patients (42 %) with a positive cystic duct margin had residual disease in the resected EBD [41]. Routine EBD resection for every GBC patient, even without evidence of tumor involvement, is recommended by some Japanese surgeons [63]. This approach is supported by a histological study that found cancer cells in the EBD in 19 of 44 (43 %) non-jaundiced patients with T2/3 GBC [64]. On the other hand, EBD resection and preservation were compared in a retrospective nationwide Japanese study including 838 T2–4 GBC patients [65]. These patients had no macroscopic involvement of the hepatoduodenal ligament and underwent an R0 resection with or without EBD resection. No difference was found in survival between EBD resection and preservation for any subgroup of T stage or N stage. A theoretical advantage of routine EBD resection is that it facilitates regional lymphadenectomy and avoids ischemia of the EBD. However, no difference in lymph node count was found between patients with and without bile duct resection [66]. Although the actual benefit of routine EBD resection remains disputed, the high complication rate is well established in both Western and Asian series. In a series of 104 GBC patients, 33 % of the patients undergoing an EBD resection had a complication that required re-intervention or resulted in permanent disability or death, versus 13 % of patients who had no EBD resection [66]. A postoperative biliary anastomotic leak may result in sepsis and death. In the long term, biliary strictures and recurrent cholangitis render the patient a “biliary cripple”[67]. The weight of evidence would support resection of the EBD only if involved with tumor or it is otherwise unavoidable in order to achieve an R0 resection.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree