Emerging from a largely cytokine-based era, the last several years have witnessed a dramatic change in the therapeutic landscape of renal cancer. Molecularly targeted and antiangiogenic agents now form the backbone of most therapeutic strategies for patients with advanced renal cell carcinoma (RCC). Although the next few years may not see such broad paradigm shifts, there remains significant room for improvement in the care of patients with RCC. This review discusses challenges that face physicians and researchers as well as innovations that may contribute to improving the therapeutic outcomes for patients with RCC.
Novel therapeutic strategies incorporating approved agents
In 2010, an estimated 58,000 new cases of renal cell carcinoma (RCC) were diagnosed in the United States and approximately 13,000 individuals died of this disease. Renal cancer is made up of several different types of cancer, each of which has distinct molecular underpinnings and a different response to treatment. For clear cell RCC (the most common variant), 3 potentially distinct targets and related therapeutic approaches are available: immunotherapy, vascular endothelial growth factor (VEGF) (antiangiogenic therapy), and mTOR (mammalian target of rapamycin) (targeted therapy). Several therapies have received approval for the treatment of advanced RCC. Table 1 displays an algorithm for the current use of the agents. Application of these treatment options has improved the overall survival (OS) for patients with advanced RCC from a median of 10 months in 1999 to in excess of 2 years. Nonetheless, for patients who either present with or develop metastatic disease the expected 5-year survival rate is still only approximately 10%. Although there are ongoing efforts to further clarify what can be expected therapeutically from these existing agents, much effort will be focused on identifying potential opportunities to enhance the efficacy of approved therapies either through: (1) use of novel dosing or scheduling strategies; (2) defining optimal treatment sequencing; (3) identifying opportunities for successful application of combination therapy; and (4) developing predictive biomarkers of response to particular therapies. It is hoped that such efforts will not only lead to improved clinical outcomes using currently available therapies but will also highlight obstacles that can be overcome only with the identification of novel therapeutic targets and strategies.
| Setting | Phase III Trials | Alternatives |
|---|---|---|
| First-line therapy | ||
| Good or intermediate risk a | Sunitinib or bevacizumab plus IFN or pazopanib | High-dose IL-2, IFN-α |
| Poor risk a | Temsirolimus | Sunitinib |
| Second-line therapy | ||
| Previous cytokine | Sorafenib | Sunitinib or bevacizumab |
| Previous VEGFR inhibitor | Everolimus | Other VEGF-targeted therapy |
| Previous mTOR inhibitor | Clinical trials | Clinical trials |
Novel Dosing or Scheduling Strategies
The determination of optimal dose for VEGF pathway inhibitors has been based on phase I trials. However, the optimal dose derived from dose-limiting toxicities observed in a handful of patients may not be the optimal dose for each individual patient. For example, studies with sorafenib have suggested that some patients at time of resistance may respond to an increase in sorafenib dose from 400 to 600 mg twice a day and that in patients in whom sorafenib dose is routinely escalated every 4 weeks, if tolerated, tumor response rates may be considerably higher. Thus, it is conceivable that some patients who experience disease progression on currently available agents may be receiving less than their optimum dose.
This conclusion is supported by investigations into the relationship between sunitinib exposure and efficacy end points in patients with advanced solid tumors including metastatic RCC. Houk and colleagues determined that higher steady-state area under the curve (AUC) of total drug (sunitinib and its active metabolite SU12662) was significantly associated with longer time to tumor progression (TTP) and improved OS; as well as higher probability of tumor response. These results suggest that maintaining the highest tolerable dose of sunitinib may be important for maximum efficacy. Furthermore, because there is likely wide pharmacokinetic variation between individuals, to maintain optimal drug exposure, it is conceivable that dosing patients based on blood levels similar to antibiotics or anticonvulsants may prove more efficacious than using the current standard daily dose of 50 mg for all patients. Studies aimed at dosing patients based on drug concentration (AUC) are being planned and may ultimately alter the dosing paradigm for sunitinib and other VEGF pathway blockers.
The development of either systolic or diastolic hypertension has also been shown to correlate with response to VEGF pathway inhibitor therapy. Houk and colleagues also established a correlation with total drug concentration (sunitinib and its metabolite) and diastolic blood pressure, which suggests that hypertension might represent a surrogate marker for VEGF receptor (VEGFR) tyrosine kinase inhibitor (TKI) blood levels and that escalating drug dose until hypertension is observed might represent an alternative means of ensuring sufficient dosing. However, this pharmacodynamic parameter may be confounded by the additional variable of VEGF/VEGFR polymorphisms which might decouple the direct correlation between blood level and pharmacodynamic effect (hypertension and/or response). The clinical usefulness of this strategy is being investigated in a trial in which patients without hypertension after an initial 4 weeks of axitinib therapy are randomly assigned to receive either additional axitinib or placebo (NCT00835978). This trial will also provide the opportunity to further establish any linkage between axitinib blood levels, hypertension (a potential pharmacodynamic marker), and tumor response.
An alternative strategy for achieving higher peak drug concentrations and minimizing toxicity is to use an intermittent treatment schedule. The effect of sunitinib treatment schedule was recently examined in a phase III trial. The standard sunitinib treatment schedule (50 mg/d for 4 weeks followed by 2 weeks off treatment) was compared with continuous daily treatment (37.5 mg/d) in 292 patients with advanced RCC. Preliminary results showed a trend toward inferior TTP with the continuous dosing schedule (median 7.1 vs 9.9 months, hazard ratio 0.77, 95% confidence interval 0.57–1.04). OS and adverse event profiles were similar for the 2 regimens. Whether this finding represents a dose (pharmacokinetic) effect or a schedule effect (delaying resistance development by providing frequent treatment breaks), and how these results might influence treatment schedule considerations for other VEGFR inhibitors in patients with RCC, is an area of potential future investigation.
The Role of Sequential Versus Combination Therapy
The availability of multiple treatment strategies for patients with advanced RCC has engendered considerable interest in treatment sequencing approaches and combination regimens. Early data supported the notion that both VEGF pathway inhibitors and mTOR-targeted therapies were active after disease progression on cytokine-based therapy. The RECORD1 trial firmly established that everolimus was more active than placebo in patients whose disease had progressed after VEGFR TKI (sunitinib, sorafenib, or both) therapy. However, a small retrospective analysis suggested that high-dose (HD) IL-2 administered after sunitinib or sorafenib resulted in an unexpectedly high rate of cardiac toxicity and produced no tumor responses, suggesting that if HD interleukin 2 (IL-2)-based immunotherapy is to be considered, it might best be used as the initial treatment. Furthermore, more recent data suggested that VEGF pathway inhibitors may have activity after disease progression on another VEGF (or even the same) pathway inhibitor. For example, sunitinib produced tumor responses in 23% of patients after progression on bevacizumab and axitinib showed similar activity in patients showing disease progression after sorafenib. Thus, the value of switching to an mTOR inhibitor after disease progression on a VEGF pathway inhibitor versus switching to another (perhaps more potent) VEGF pathway inhibitor remains to be determined. Although this specific question is being partially studied in a randomized phase III trial comparing temsirolimus with sorafenib in patients whose disease has progressed on sunitinib, such decisions will likely need to be individualized. For example, in patients who show disease progression without significant toxicity on a particular VEGF pathway inhibitor, dose escalation may be indicated, whereas for patients who have experienced a response to a VEGF pathway blocker, but then show disease progression after dose reduction because of toxicity, switching to a different VEGF pathway blocker may restore disease sensitivity. Switching to an mTOR inhibitor might be the treatment of choice for patients whose disease is upfront resistant to VEGF pathway blockade or has progressed after multiple (or the most potent) VEGF pathway blocker(s). However, a retrospective study showed that the institution of mTOR inhibitors may not be better than switching to alternate VEGF pathway inhibitors in patients with tumors that are primarily refractory to anti-VEGF therapy, suggesting that a different approach may be necessary for such patients.
An alternative strategy for maintaining disease control or enhancing treatment effect is to administer active treatments in combination. Combination therapy strategies could include the combining of molecularly targeted agents with immunotherapy (interferon [IFN] or HD IL-2), combining a VEGF blocking agent (bevacizumab) with a VEGFR TKI (vertical blockade) or combining a VEGF pathway inhibitor with an mTOR or other pathway inhibitor (horizontal blockade). All of these approaches have been tested clinically, with varying results.
Vertical blockade with either sorafenib or sunitinib combined with bevacizumab has shown impressive antitumor activity but disappointing toxicity. For example, the combination of sorafenib and bevacizumab produced tumor responses in 52% of patients with advanced RCC; however, the regimen also produced enhanced toxicity, necessitating drastic reductions in both agents to maintain its tolerability. Similarly the combination of sunitinib and bevacizumab produced responses in more than 50% of patients but also produced a microangiopathic hemolytic anemia syndrome with associated kidney failure and neurologic toxicity in patients who received extended therapy. This syndrome, which is similar to preeclampsia (a disease attributed to tissue VEGF starvation caused by high levels of circulating soluble VEGFR), may show the limits of tolerability of prolonged and profound VEGF pathway blockade.
Compared with the vertical blockade, horizontal blockade strategies have in general been more tolerable. The combination of bevacizumab and erlotinib was well tolerated, but proved no more effective than bevacizumab alone. In contrast, the combination of either temsirolimus or everolimus with bevacizumab was not only tolerable at the full doses of each agent but also produced response rates that appeared to be superior to what would be anticipated with mTOR inhibition alone. The value of these mTOR + VEGF inhibitors combinations is being explored in a series of randomized phase II and III trials. However, the results of the initial trial were disappointing because the combination of bevacizumab and temsirolimus appeared more toxic, but not more efficacious than either sunitinib or bevacizumab plus IFN in treatment-naive patients with advanced RCC.
Perhaps the most encouraging results have been seen with the combination of VEGF pathway inhibitors and immunotherapy. Both the AVOREN trial and the CALGB-led Intergroup Trial established a benefit for the addition of bevacizumab to IFN-α in terms of response rate and median progression-free survival (PFS). The number of patients experiencing a complete or durable response remained small in the combination arm and lacking this or a bevacizumab alone arm, no information is available to firmly establish a contribution of IFN to the combination. Similar results were seen with the combination of bevacizumab and HD IL-2. In this phase II trial, the activity of the combination appeared to be additive, with 8% of patients achieving a complete response (typical of HD IL-2) and a median PFS of 9 months similar to what is seen with bevacizumab alone.
When considering the clinical usefulness of these combinations, one must keep in mind several principles: (1) lowering the dose of an active agent to accommodate the toxicity of a less active agent (as in the temsirolimus plus IFN combination) might diminish efficacy; (2) inhibition of additional pathways (at least in some tumors) may produce countervailing effects or just additional side effects for the patient; and (3) because of the additional toxicity and expense associated with the administration of 2 agents simultaneously, the results of horizontal blockade should be significantly better than the administration of the 2 component agents in sequence to be considered useful.
When viewed in their entirety, the results with combination regimens must be viewed as a major disappointment. Future approaches could be more fruitful by focusing on (1) combinations that might yield durable responses (ie, combinations with immunotherapy); (2) studying combinations that involve the potential for maintenance of VEGF blockade at the time of resistance (eg, the CALGB trial comparing everolimus vs everolimus + bevacizumab in patients after disease progression on sunititinib or sorafenib); or (3) devising combinations based on understanding of mechanisms of resistance/escape that therefore have increased likelihood of delaying the onset of resistance. Several potential mechanisms for resistance and, therefore, promising pathways to be targeted in combination regimens are discussed in a later section of this review.
Patient Selection Strategies
The availability of approved agents with distinct mechanisms of action (immunotherapy, mTOR, and VEGF pathway inhibitors) has complicated treatment decisions for patients with advanced RCC. Although randomized phase 3 trials can provide guidance for the average patient in a specific clinical situation, individual patients and tumors have distinct characteristics that may greatly influence their response to different treatments. Identifying the optimal treatment of an individual patient has become an important goal of current investigation. Many recent clinical trials have stratified patients by clinical prognostic factors developed by Motzer and colleagues from analysis of patients treated with IFN-α. More recently, algorithms have been developed that identify prognostic factors for patients receiving VEGF pathway targeted therapies (reviewed by Daniel Heng elsewhere in this issue). These novel algorithms have yet to be used prospectively and given that they overlap substantially with the prognostic factors identified for patients receiving IFN therapy, may be a better indicator of general tumor biology than the impact of specific treatment (or treatment approach) on a particular patient population. Although such algorithms may be useful, the goal remains to be able to choose treatment strategies based on models that take into consideration not just clinical factors but also the pathologic, molecular, and biologic features of the tumor.
Immunotherapy
Although recent phase 3 trials have established the superiority of VEGF-targeted and mTOR-targeted therapies over IFN-based immunotherapy in patients with advanced RCC, a subset of patients clearly exist who develop significant clinical benefit from immunotherapy and for whom omitting this treatment option might greatly compromise their long-term treatment outcome. This situation is particularly true for HD IL-2 therapy, which has been shown in 2 randomized phase 3 trials to produce higher response rates and more durable complete responses than lower-dose cytokine regimens and based on trial comparisons more off-treatment sustained responses than are seen with the VEGF and mTOR pathway inhibitors. Recent studies suggest that in the current era the response to HD IL-2 exceeds 25% with at least 10% of patients showing complete responses that last in excess of 2 years (see article by David F. McDermott elsewhere in this issue). Thus, cytokine therapy, particularly HD IL-2, remains a reasonable initial treatment option for some patients with metastatic RCC. However, given the toxicity associated with HD IL-2, identifying predictors of response (or resistance) to this therapy and thus limiting its use to those most likely to benefit remains a high priority.
Efforts to identify predictive factors for response to HD IL-2 have focused on disease, immunohistochemistry (IHC), and gene expression patterns. Past data suggest that responses to immunotherapy are almost exclusively seen in patients with clear cell RCC. Although initial efforts suggested that further subdivision of clear cell RCC based on percentage of granular or papillary features might be useful, this approach was not validated in a recently reported prospective trial. Several retrospective analyses reported that IHC staining for carbonic anhydrase IX (CAIX), an enzyme the expression of which is mediated by the hypoxia-inducible factor (HIF) transcriptional complex, was associated with improved survival and a higher objective response rate in IL-2–treated patients ; however, this predictive biomarker has yet to be validated prospectively. More recent studies with array-based comparative genomic hybridization showed that tumors from complete responders to HD IL-2 had fewer whole chromosome losses than nonresponders. The concentration of losses in sections of chromosome 9p (65% in nonresponders vs 0 in complete responders), which encompasses genes such as CAIX, pS6, and B7H1, adds some potential mechanistic significance to this observation. Studies in RCC and other tumors have suggested that immune cell infiltration (dendritic cells, natural killer cells, CD8 T cells) is associated with improved disease outcome as well as response rates to immunotherapy. This research suggests that an extant, but blunted, immune recognition of the tumor may be a prerequisite for response to immunotherapy. Studies of patterns of tumor immune infiltrates using IHC, reverse transcription-polymerase chain reaction, and the examination of host DNA for polymorphisms associated with autoimmunity, are under way. Although the findings will likely require prospective validation, perhaps as part of the IL-2 Select trial, if confirmed they may provide powerful tools for clinicians in selecting patients not just for IL-2 therapy but also for novel immunotherapies in development. In addition, such biomarkers might provide clues to understanding the sensitivity of RCC, and possibly other tumor types, to immunotherapy.
mTOR inhibitors
Although the clinical benefit of HD IL-2 seems limited to patients with clear cell RCC, this may not be the case with inhibitors of the mTOR pathway. A subsequent analysis of the randomized phase 3 trial of temsirolimus versus IFN showed that the median OS of patients with non–clear cell RCC (75% of whom had the papillary subtype) was 11.6 months in the temsirolimus group versus 4.3 months in the IFN group. This differential contrasts with the minor improvement over IFN associated with temsirolimus therapy in patients with clear cell RCC. The preferential activity of temsirolimus in non–clear cell RCC also contrasts with what is seen with the VEGFR antagonists sorafenib and sunitinib, both of which have only limited activity against these RCC variants. These observations suggest that the mechanism of action of mTOR inhibitors may be distinct from those of agents primarily inhibiting VEGFR signaling in the tumor endothelium. During the clinical development of mTOR inhibitors, the antagonism of HIF-1α expression by these drugs has often been proffered as a logical explanation for their clinical efficacy in RCC. However, these observations highlight the need for further investigation into the mechanism of action of mTOR inhibitors and the need to identify molecular features that may predict response to these agents.
Preliminary efforts to identify such predictive biomarkers have focused on pathologic surrogates of the basal activation status of the presumptive molecular targets of mTOR inhibitors. A small retrospective analysis of pretreatment tumor specimens from a subset of patients with RCC treated with temsirolimus as part of the randomized phase 2 trial reported an association of high expression of either phospho-Akt or phospho-S6 ribosomal protein, substrates upstream and downstream of mTOR, respectively, with objective response to temsirolimus. In contrast, no apparent correlation was found of CAIX or PTEN expression. A larger analysis of tumor specimens from patients treated with temsirolimus as part of the randomized phase 3 trial also found no correlation between tumor PTEN expression and either tumor response or OS or PFS. In addition, no such correlations were observed with baseline HIF-1α expression. Although the stability of certain phosphoproteins, in particular phospho-Akt, has been called into question, phospho-S6 seems to be a promising potential predictive biomarker for response to mTOR inhibitors. However, this marker still requires validation in larger retrospective analyses and/or prospective studies.
Future efforts to identify predictive biomarkers of response to mTOR inhibitors must be guided by insights into the mechanism of both response and resistance to mTOR inhibitors. For example, overexpression of eurokaryotic initiation factor 4E (eIF4E) would be expected to make a cell relatively resistant to growth inhibitory efforts of mTOR inhibition. The frequency of basal overexpression of eIF4E in RCC remains to be investigated. Recent studies have also reported somatic activating mutations in mTOR in some RCC. The extent to which such mutation or other mutations in regulators of the AKT/mTOR pathway (TSC1/TSC2; FOX0; PI3K, LKB1, or REDD1) occur needs to be determined along with the assessment of their potential impact on tumor responsiveness to mTOR-targeted therapy. Although the groups of patients deriving clinical benefit from mTOR inhibitors and VEGF-targeted therapies likely overlap to some degree, incorporation of molecular and pathologic features of the tumor into selection schemes will be important to help direct the use of mTOR inhibitors in the adjuvant, first-line, sequential, and combinational therapy for patients with RCC.
VEGF pathway inhibition
As noted earlier, clinical factors predictive of poor outcome with VEGF-targeted therapies tend to overlap those reported for IFN treatment. Heng and colleagues report in a multivariable analysis that 4 of the 5 adverse prognostic factors in the Memorial Sloan Kettering Cancer Center model (time from diagnosis to current treatment less than 1 year, hemoglobin <lower limit of normal, corrected serum calcium > upper limit of normal, and Karnofsky performance scale score <80%), were also independent predictors of short survival in patients receiving VEGF pathway inhibitors. In addition, increased baseline platelet and neutrophil counts were associated with poor prognosis. From these factors, 3 prognostic subgroups were identified with a median OS of 27 months, 24 months, and 8.8 months, respectively. The clinical usefulness of these factors is compromised by the inability to sort out whether they are truly predictive or simply prognostic and because most patients show some degree of treatment shrinkage with VEGF pathway inhibition, making it harder to identify patients with upfront treatment resistance.
Efforts to identify molecular factors associated with poor outcome with VEGF pathway therapy have also begun. High baseline VEGF levels have been associated with poor outcome in both the TARGETs and Avoren trials ; however, patients with high and low baseline VEGF levels benefited from sorafenib and bevacizumab in terms of PFS. This finding suggests that serum VEGF, although having prognostic significance, is not a predictive biomarker for benefit from VEGF-targeted therapy. Rini and colleagues suggested that lower baseline levels of sVEGFR-3 and VEGF-C were associated with longer PFS and better tumor response in patients receiving sunitinib after disease progression of bevacizumab. However, this work requires validation. Patel and colleagues reported that the level of HIF-2α expression in pretreatment tumor specimens by Western analysis correlated directly with response to sunitinib treatment ( P <.0001). Additional studies are needed to confirm the predictive value of HIF-2α for sunitinib and other VEGF-targeted agents in patients with metastatic RCC. However, given that HIF-2α expression seems to be a near universal feature of von Hippel-Lindau (VHL) null clear cell RCC, whereas HIF-1α expression is lost in up to 25% of tumors, determining the differential effect of HIF-1α expression on treatment outcome may ultimately prove more useful.
Efforts to determine the predictive value of tumor VHL gene inactivation on response of patients with advanced RCC to VEGF-targeted agents have yielded conflicting data. Patients with tumors containing VHL inactivation ( VHL mutated or methylated) had a response rate of 41% compared with 31% for patients with tumors with wild-type VHL ( P = .34). Patients treated with sorafenib and bevacizumab responded only if their VHL gene was inactivated in contrast to patients treated with sunitinib or axitinib who experienced responses irrespective of the VHL gene status of their tumor. In other studies, tumor VHL status did not seem to be predictive of response to axitinib (N = 13) or pazopanib (N = 78). Although data concerning the impact of tumor VHL gene status on PFS and OS in patients receiving VEGF-targeted therapy may be more clinically relevant, these results are nonetheless of potential therapeutic significance. They suggest either that sunitinib, axitinib, and possibly pazopanib have additional non– VHL -related antitumor effects in RCC or that the VHL /HIF/VEGF pathway remains an important target in VHL wild-type RCC but requires more potent inhibition for clinical activity to be manifest. More recent genotyping studies asserting that more than 90% of clear cell RCC have dysfunctional VHL suggest that false-negative genotyping might contribute to the apparent lack of predictive value tumor VHL inactivation for response to VEGF pathway therapy. Alternatively, this lack of association might indicate that other molecular features (eg, baseline IL-8 expression, fibroblast growth factor [FGF] upregulation) may be more critical contributors to the resistance of clear cell RCC to VEGF pathway therapy.
Thus, the investigation of treatment selection factors for patients with advanced RCC remains a work in progress. Although some information is available, considerably more research is needed to identify and validate selection factors for particular treatment approaches. In the future, these approaches are likely to include not only clinical features and blood-based and tissue-based biomarkers but also sophisticated functional imaging studies and increasing assessment of both tumor and germline polymorphisms. Because identification of the optimal first-line treatment of a particular patient is a prerequisite to determining the optimal second-line therapy, treatment sequence, or, more importantly, determining those patients who need different treatment approaches altogether, initial treatment selection research remains a priority.
Novel Therapeutic Agents
In addition to novel therapeutic strategies, the landscape of RCC will also be altered by the emergence of many novel therapeutic agents. Some of these new drugs represent the next generation of agents directed against already established therapeutic targets such VEGFR2 and mTOR. Others will be drugs with novel therapeutic targets in some cases identified through studies into the mechanisms of resistance and response to available therapies.
Novel Agents Targeting VEGF Signaling
As discussed earlier, the development of agents disrupting VEGF signaling has been a major therapeutic breakthrough for patients with advanced RCC. First-generation TKI with activity against VEGFR2, such as sunitinib and sorafenib, have emerged to form the backbone of advanced RCC therapy. However, these agents have certain limitations. Responses to these drugs are typically neither complete nor durable off therapy, and their usefulness is often limited to moderate disease regression and significant, but transient, prolongation of disease stability. Moreover, the continuous administration of these agents is limited in some patients by toxicity, requiring holding of medication, dose reduction, and even treatment cessation.
The next generation of VEGF-targeted TKI may prove superior to first-generation TKI in terms of both efficacy and side effect profile. As shown in Table 2 , agents such as axitinib and tivozinib boast significantly greater potency against VEGFR compared with earlier-generation TKI. Moreover, the potency of these agents is relatively more focused on the VEGF receptors than other tyrosine kinase receptors such as C-Kit and platelet-derived growth factor receptors (PDGFRs). As these agents develop, the hope is that these molecular characteristics will lead to greater efficacy coupled with less toxicity compared with first-generation TKI.
| Agent | IC 50 (nM) | ||||
|---|---|---|---|---|---|
| VEGFR-1 | VEGFR-2 | VEGFR-3 | c-KitR | PDGFR-β | |
| Tivozinib | 0.2 | 0.2 | 0.2 | 1.6 | 1.7 |
| Axitinib | 1.2 | 0.3 | 0.3 | 1.6 | 1.7 |
| Sunitinib | 2 | 10 | 17 | 10 | 8 |
| Pazopanib | 15 | 8 | 10 | 2.4 | 14 |
| Sorafenib | NA | 90 | 20 | 68 | 80 |
Preliminary clinical results have been promising. In a phase II randomized discontinuation trial of tivozinib in patients with advanced RCC, the median PFS of all patients was 11.8 months and the objective response rate 27%. However, in patients with clear cell RCC who had undergone nephrectomy, the PFS was 14.8 months and the objective response rate was 32%. Tivozinib is being studied in a randomized phase III trial versus sorafenib in patients with advanced RCC. Likewise, axitinib has shown robust clinical activity in patients with advanced RCC in multiple clinical settings. Axitinib was initially evaluated in a phase II trial in patients with cytokine-refractory RCC. The median TTP of patients treated with axitinib was 15.7 months and the objective response rate 44.2%. Axitinib was subsequently studied in patients who had failed previous sorafenib and again it showed activity. The median PFS of patients treated with axitinib was 7.1 months and the objective response rate 22.6%. Based on these results, axitinib is being assessed in a randomized phase III trial versus sorafenib in patients with advanced RCC who have failed sunitinib (Axis trial). Although the results from this trial have yet to be reported, preliminary press releases from Pfizer Pharmaceuticals indicate that the primary end point of this trial (superior PFS) has been met. Positive results from these 2 ongoing phase III trials will likely have an immediate impact on the second-line therapy for RCC and possibly change front-line therapy as well.
Novel Agents Targeting mTOR
Inhibitors of mTOR represent a second class of molecularly targeted agents that have shown activity in patients with advanced RCC, and 2 such agents, temsirolimus and everolimus, are now approved by the US Food and Drug Administration (FDA) for the treatment of patients with RCC. Similar to VEGF-targeted TKI, significant responses to these agents are infrequent and typically short-lived and all patients treated with these drugs eventually develop progressive disease. The efficacy of these allosteric inhibitors of mTOR may be limited in part because they primarily inhibit the function of TORC1, the complex including mTOR and raptor (regulatory associated protein of TOR), and have less activity against TORC2, the complex including mTOR and rictor (rapamycin insensitive companion of TOR). Although the clinical activity of mTOR inhibitors in RCC was largely discovered empirically, the ability of the rapalogues to attenuate HIF-1α gene expression by reducing both mRNA and protein stabilization has long been proposed as a potential mechanism of action of these agents in RCC. This situation may be particularly true in the clear cell variant, most of which possess biallelic alterations in the VHL gene, resulting in the accumulation of HIF-1 and HIF-2 and the subsequent activation of their target genes, including VEGF , PDGF , TGF- α, and CXCR4 . However, although HIF-1α and HIF-2α are known to have overlapping effects on gene expression, HIF-2α has been argued by many to be the more relevant HIF with respect to the development and progression of RCC. It has recently been shown that a substantial fraction of VHL−/− RCC express HIF-2α only. Recent studies have also suggested that the translation of HIF-2α is more dependent on the activity of TORC2 and largely independent of the TORC1 activity. Together these findings highlight the potential therapeutic value of simultaneously inhibiting both TORC1 and TORC2 in RCC.
A new generation of mTOR inhibitors is under development that are not allosteric inhibitors of mTOR but rather bind directly to the adenosine triphosphate-binding domain of mTOR, thereby inhibiting the function of both TORC1 and TORC2. The aforementioned ability to suppress HIF-2α expression would not be the only theoretic advantage of such agents in RCC. One of the primary activities of TORC1 inhibitors is believed to be the dephosphorylation and activation of eukaryotic translation initiation factor (eIF4E)-binding proteins (4EBPs), which function to sequester and block eIF4E from performing cap-dependent translation of certain difficult-to-translate mRNAs such as those of VEGF , c yclin D , c-Myc , and survivin . However, the phosphorylation of 4E-BP1 has long been recognized to be less responsive to rapalogues than that of the S6 ribosomal protein and the suppression of 4E-BP1 phosphorylation by rapamycin is reversed within a few hours of drug exposure. The active site inhibitors of mTOR would therefore also have the added advantage of achieving more effective suppression of 4E-BP1 phosphorylation and therefore attenuation of cap-dependent translation. Whether these theoretic advantages will translate into superior clinical activity will be an area of active investigation in the coming years.
Novel Immunotherapy Approaches
As discussed earlier, immunotherapy with HD IL-2 maintains the advantage over other therapies of offering a higher percentage of durable responses for patients with advanced RCC. However, the possibility of achieving these durable responses must be balanced with the potential toxicity of HD IL-2. Nonetheless, it must be conceded that at least a subset of RCC remain responsive to immune-stimulating agents. Novel immunotherapy approaches will seek to further exploit the immune-responsiveness of RCC and avoid the well-described toxicities of HD IL-2. Although this topic is covered in more detail in the article by Rosenblatt and McDermott elsewhere in this issue, a few novel immune targets are worthy of special mention.
Although the exact mechanism by which HD IL-2 exerts its antitumor activity remains unknown, it is widely believed that immune-stimulating agents result in the generation, activation, or expansion of a population of T lymphocytes that recognize antigens expressed by RCC. Improved understanding of various mechanisms by which T-cell activation can be positively or negatively regulated has led to the development of agents that can activate the immune system by modulating costimulatory signals on T cells. These novel immune therapies include antibodies against CTLA-4 and PD-1.
CTLA-4 is a costimulatory receptor expressed on the surface of activated T lymphocytes that, when activated, prevents further lymphocyte proliferation. Preventing engagement of this inhibitory signal has emerged as a strategy to augment or release an antitumor immune response. Currently approved by the FDA for the treatment of patients with metastatic melanoma, ipilimumab, an antibody directed against CTLA-4, has seen a limited evaluation in patients with metastatic RCC. In a phase II trial run by the National Cancer Institute Surgery Branch, 2 cohorts of patients with advanced RCC received 2 different dosing schedules of ipilimumab: a 3-mg/kg loading dose followed by either 1-mg/kg or 3-mg/kg maintenance doses every 3 weeks. Of the 21 patients receiving the 1-mg/kg maintenance dose, 1 patient (4.7%) experienced a partial response. Of 40 patients treated with the 3-mg/kg maintenance dose, 5 (12.5%) experienced partial responses. Responses were observed in patients who had failed previous HD IL-2, suggesting that there is no clear cross-resistance. Given its recent FDA approval for melanoma, ipilimumab will likely see further evaluation in RCC in the near future.
PD-1 is another costimulatory receptor that is expressed on activated T lymphocytes and when activated downmodulates T-cell function. A major ligand for PD-1, B7-H1 (PD-L1), was shown to be overexpressed in many RCC, and greater expression was associated with worse prognosis. It has been suggested that expression of B7-H1 by RCC is a strategy to evade immune detection and activation. Efforts are under way to block PD-1 activation as a means of reactivating an antitumor immune response. MDX-1106 is a monoclonal antibody directed against PD-1 that was recently assessed in a phase I trial including many patients with advanced RCC. MDX-1106 was administered in doses of 1, 3, and 10 mg/kg given every 2 weeks. Of 16 patients with RCC treated at various doses, 5 patients (31%) achieved objective responses, including 1 complete response. This promising activity coupled with a mild toxicity profile has prompted the rapid assessment of MDX-1106 in multiple phase II trials in patients with advanced RCC. Over the next several years, agents such as ipilimumab and MDX-1106 will likely be assessed, along with HD IL-2, in various sequences and combinations with the goal of achieving higher rates of durable responses than what is possible with currently available therapies.
Therapeutic Targets Identified by Resistance Mechanisms
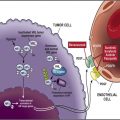
Elucidating the mechanism by which RCC develops resistance to VEGF-targeted therapies is perhaps the most critical priority for RCC research. Because complete responses to these antiangiogenic agents remain rare, it is clear that most RCC possess the intrinsic ability to survive the immediate insult of hypoxia and nutrient deprivation induced by the reduction in tumor vasculature and are able to eventually activate escape pathways to restore tumor perfusion. It is hoped that the identification of critical signaling pathways that mediate both the intrinsic resistance to antiangiogenic agents and the delayed restoration of microvasculature angiogenic escape will identify novel therapeutic targets in RCC. Efforts directed toward this end have already identified several such targets.
Angiopoietins
The angiopoietins (Angs) are ligands for the endothelial-specific tyrosine kinase receptors Tie-1 and Tie-2 and are believed to play a critical role in vessel maturation and the maintenance of vessel integrity. Ang-1 and Ang-2 are the most frequently implicated Angs in tumor angiogenesis and are specific ligands for Tie-2. Ang-2 is highly expressed by the endothelial cells of many tumors and is believed to act in tandem with VEGF to promote tumor-mediated angiogenesis. Activation of the Ang/Tie-2 axis had been implicated in both basal angiogenesis in response to hypoxia in RCC and in vascular survival in the setting of VEGF blockade. AMG-386 is an antiangiopoietin peptibody (an Ang-recognizing peptide fused to the Fc region of an antibody) that can inhibit the interaction between Ang-1 and Ang-2 with Tie-2. Given the possible synergistic relationship between VEGF and Ang-2 in promoting angiogenesis, the combination of AMG-386 with VEGF-targeted agents has emerged as a therapeutic strategy in RCC. Although a randomized phase II trial in patients with advanced RCC failed to show a prolongation in the PFS in patients treated with sorafenib in combination with 2 different doses of AMG-386 compared with placebo, a higher objective response rate was noted in the AMG-386–containing arms. A single-arm phase II trial combining sunitinib with AMG-386 in patients with treatment-naive or cytokine-refractory metastatic RCC is ongoing and results are expected soon.
IL-8
IL-8 is a member of the CXC family of chemokines and is known to be a potent proangiogenic factor. Angiogenesis mediated by IL-8 may occur in a VEGF-independent manner as suggested by Mikukami and colleagues in a colon cancer xenograft model in which VEGF signaling was opposed. It was recently shown in multiple RCC xenograft models that IL-8 secretion was upregulated in tumors treated with sunitinib coincident with restoration of microvasculature heralding treatment resistance. Concurrent treatment of the same xenograft models with sunitinib and a neutralizing antibody against IL-8 resulted in the restoration of sensitivity of the tumors to sunitinib. In patients with clear cell RCC resistant to sunitinib, expression of IL-8 was found to be increased in their archived tumor specimens, suggesting that IL-8 may play a role in upfront resistance to VEGF-targeted agents in addition to a role in delayed resistance. Efforts to develop therapeutic strategies to inhibit IL-8–mediated signaling are under way and there will be great interest in examining the additive effects of these agents in combination with VEGF-targeted therapies in RCC in the years to come.
FGF
FGF is a soluble growth factor produced by both tumors and tumor microenvironment, which are also potent angiogenic factors signaling through tyrosine kinase receptors. FGF-2 was initially found to be upregulated by Casanovas and colleagues in an islet cell tumor model of acquired resistance to VEGF-targeted therapy. Welti and colleagues recently showed that FGF-2 can oppose the antiangiogenic effect of sunitinib by directly stimulating a proangiogenic signal in endothelial cells. This effect was opposed by concurrent treatment with an inhibitor of the FGF receptor tyrosine kinase. Furthermore, greater than 50% of human RCC specimens were found to strongly express FGF-2 in both the tumor cell and tumor endothelium. Taken together, these findings suggest that there may be some value to the simultaneous targeting of both FGF and VEGF signaling. There are now several multitargeted TKIs in various stages of clinical development with broad activity against VEGFR-1, VEGFR-2, PDGFR-β, and FGF receptor 1. Many of these agents, such as E7080 (Eisai Pharmaceuticals, Tokyo, Japan) and Dovitinib (TKI258, Novartis Pharmaceuticals, Basel, Switzerland) are in active clinical study in RCC, and the results of these studies will inform the value of this therapeutic approach.
Emerging Novel Therapeutic Targets
In addition to the therapeutic targets identified by studies into resistance to VEGF-targeted therapy, several other molecular targets will likely be actively investigated in RCC in the upcoming years. Those targets closest to therapeutic investigation in the near future are discussed in the following sections.
Phosphoinositide 3-kinase/Akt
The aforementioned clinical activity of the rapalogues in RCC has highlighted the potential relevance of the mTOR pathway to RCC pathogenesis and progression. The kinase activity of mTOR is regulated by a complex system of upstream and downstream elements including phosphoinositide 3-kinase (PI3-K), Akt, and the tumor suppressor PTEN homolog. The PI3-K/Akt pathway regulates the function of a broad array of proteins involved in cell growth, proliferation, motility, adhesion, neovascularization, and cell death. One of the potential mechanisms of resistance to RCC to TORC1 inhibitors is through the feedback activation of the PI3-K/Akt. Treatment with TORC1 inhibitors has been shown in some cases to result in the activation of PI3-K through a feedback loop involving the insulinlike growth factor 1 receptor. TORC1 inhibitors also can activate Akt directly through the derepression of TORC2, which results in TORC2-mediated phosphorylation of Akt on Ser 473 . PI3-K/Akt signaling activates an array of kinases, transcription factors, and other proteins besides mTOR that promote cell growth and survival. This prosurvival effect is primarily executed by Akt through a variety of pathways including the negative regulation of factors promoting the expression of death genes (eg, inactivation of forkhead family proteins), positive regulation of prosurvival genes (eg, activation of NF-κB), direct phosphorylation of proapoptotic proteins (eg, inactivation of BAD), and regulation of the cell cycle. Disruption of any of these prosurvival signals may have therapeutic benefits that complement the effects of mTOR inhibition and enhance antitumor activity, a notion that has been supported in preclinical studies both in vitro and in vivo.
Inhibitors of PI3-K and Akt are now entering active clinical development in RCC. Because the catalytic domain of mTOR and p110α subunit of PI3-K are structurally related, multiple agents are also in development that have dual inhibitory activity against PI3-K and mTOR. In addition to being inhibitors of PI3-K, these agents have the added benefit of directly inhibiting mTOR kinase activity, thereby inhibiting the function of both TORC1 and TORC2. Preclinical studies have also supported the efficacy of these dual inhibitors in RCC. Should these agents prove superior to allosteric inhibitors of TORC1 in clinical studies, it is possible that they may replace the rapalogues in the therapeutic landscape of RCC within the next several years.
Hepatocyte growth factor/c-MET
Directly involved through germline mutation in hereditary papillary RCC, c-MET is a tyrosine kinase and protooncogene the activation of which is believed to promote tumor invasiveness. Activation of c-MET in RCC has been linked to VHL loss as well as hypoxia and may play an important role in malignant progression. Higher expression of c-MET in RCC tumor specimens has been associated with higher tumor grade and clinical stage and was also found to be an independent predictor of poor OS. Similar to many of the molecular targets mentioned earlier, c-MET has also been implicated in the development of resistance to VEGF-targeted therapy through maintenance of an alternate angiogenic pathway. Treatment of RCC xenografts resistant to sunitinib with the combination of sunitinib and a selective c-MET inhibitor resulted in significantly greater inhibition of tumor growth than treatment with either agent alone.
Inhibition of c-MET signaling is being explored clinically either through direct inhibitors of c-MET receptor tyrosine kinase activity or through disruption of the interaction of c-MET with its ligand hepatocyte growth factor (HGF) (ie, HGF antibody). These agents are now likely to be explored both as single agents and in combination with VEGF-targeted therapy in patients with advanced RCC. XL184 (Exelixis Pharmaceuticals), a potent small molecule inhibitor of both c-MET and VEGFR2, is being investigated in a phase I drug interaction study in RCC and may develop as an attractive agent for patients who have failed previous VEGF-targeted therapy.
HIF-2α
As discussed earlier, it is now largely accepted that HIF-2α is the more relevant HIF in RCC with respect to malignant transformation and progression. As the upregulation of HIF levels as a result of loss of VHL function that characterizes most clear cell RCC results in the activation of many other genes that may drive tumor survival and proliferation besides VEGF and PDGF (eg, TGFα, CXCR4), successful inhibition of HIF-2α may have broader therapeutic effects than currently available VEGF-targeted agents. However, although HIF-2α is clearly an attractive therapeutic target in RCC, there are no direct inhibitors of HIF-2α in active clinical assessment in RCC. Efforts are under way to develop such agents, and successful evaluation of these agents in RCC may represent a major therapeutic advancement in the next several years.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree