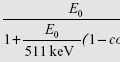1
FUNDAMENTAL PHYSICS
1.2 ATOMIC AND NUCLEAR STRUCTURE
Question 2
What is a roentgen (R) and what is it used for?
Question 3
What is the SI unit for absorbed dose?
Question 4
What is the relationship between Sievert (Sv) and rem?
The units of radioactivity are the Becquerel (Bq) and Curie (Ci). 1 Bq = 1 decay/sec.
1 curie = 3.7 × 1010 decay/sec, thus 1 mCi = 3.7 × 107 Bq.
Answer 2
A roentgen is the unit of exposure. Exposure is the measure of ionization of photon radiation in air, defined as the charge of one sign released per unit mass. 1R = 2.58 × 10−4 C/kg at standard temperature (0°C) and pressure (760 mmHg).
Answer 3
The SI unit for absorbed dose is the gray (Gy) such that 1 Gy = 1 J/kg. Rad is the former unit for absorbed dose, 1 Gy = 100 rad, and 1 rad = 1 cGy.
Answer 4
The Sv and rem measure the effect of dose on the body. It includes the biological effect of different types of radiation such as neutrons (equivalent dose), or the increased sensitivity of different organs (effective dose). The SI unit is the Sv. 1 Sv = 1 J/kg and 1 mSv = 100 mrem.
Question 6
How does a photon’s energy relate to its frequency and wavelength?
Question 7
What is the electromagnetic spectrum?
Question 8
What is the SI unit of specific activity?
The electron volt is defined as the kinetic energy given to an electron initially at rest going through a potential difference of 1 V. It is a unit of energy with 1 eV = 1.602 × 10−19 Joules (J) and 1 amu = 931 MeV. The amu represents one twelfth of the mass of a carbon-12 nucleus.
Answer 6
The energy of a photon equals its frequency (in Hz) multiplied by Planck’s constant (h = 6.26 × 10−34 J sec). Frequency (v) is related to wavelength (λ) by c = λv. The velocity (c) is the speed of light, 3 × 108 m/sec.
Answer 7
Photons travel as electromagnetic waves, described by the electromagnetic spectrum. This spectrum defines regions based on their energy (and hence wavelength or frequency). From lowest energy (and lowest frequency, highest wavelength) the spectrum starts with radio waves, then microwaves, infrared, visible, ultraviolet, X-rays, and gamma rays.
Answer 8
Specific activity is defined as a sample’s activity (A) divided by its mass (m). The SI unit for specific activity is Bq/kg. It can also be given in terms of Ci/g. A higher specific activity allows a smaller source to be used for radiation treatment. For example, the specific activity of 60Co source is 200 Ci/g and could contain 6,000 to 7,000 Ci in a 1.5 to 2.0 cm diameter 60Co source.
Question 10
What is the relationship between energy and wavelength and how can that relationship be used to calculate the energy of a photon?
Question 11
What is the Avogadro constant?
1.2 ATOMIC AND NUCLEAR STRUCTURE
Question 1
What is the charge of an electron?
Planck’s constant (h) is given as h = 6.62 × 10−34 J sec. A photon does not possess any mass or have any charge, but does possess energy which is related to the frequency (v) of the photon by the following equation, E = hv.
Answer 10
We know that c = vλ, where λ is the wavelength and c is the speed of light (3 × 108 m/sec). By using this relationship, we can determine the frequency v and then use E = hv to calculate the energy of a photon.
Answer 11
The Avogadro constant is NA = 6.022 × 1023, which is the number of atoms in a mole of a substance.
Answer 1
The charge of an electron is 1.6 × 10−19 C. A Coulomb (C) is a unit of electric charge and represents a total charge of 6.24 × 1018 electrons. In classical physics, the smallest unit of negative charge is an electron with a charge of “−1” while the proton has the smallest unit of positive charge, “+1.”
Question 3
What is the maximum number of electrons that can be placed in a shell?
Question 4
What is the charge and mass (MeV) of an electron, proton, and neutron?
Question 5
For an atom designated by the following atomic symbol: ![]() , how does one determine the atomic mass number, the number of protons, electrons, and neutrons in the atom as well as the number of nucleons?
, how does one determine the atomic mass number, the number of protons, electrons, and neutrons in the atom as well as the number of nucleons?
The electron binding energy is determined by the Coulomb attraction between the nucleus (protons) and orbital electrons (e−). The electron binding energy increases as Z increases and decreases as the distance from the nucleus increases. The binding energy of an electron is the minimum energy required to knock the electron out of the atom.
Answer 3
Electron shells are labeled starting from the inner most shell by letters or by numbers. The inner most shell is the “K Shell” followed by the L, M, and N shells, respectively. The maximum number of electrons that can be allowed in a shell is 2n2, where n is the shell number from the inner most to outer most orbital, (K = 1, L = 2). However, the outer most shell of an atom commonly referred to as the valance shell is only able to carry eight electrons.
Answer 4
An electron has a charge of −1 and a rest mass of 0.511 MeV. The mass of an electron is 1/1,836 compared to the mass of a proton. The proton has a charge of +1, and a rest mass of 938 MeV. The neutron has a charge of 0, and a rest mass of 939 MeV.
Answer 5
For a given atomic symbol, the number of protons is equal to “Z” which is also equal to the number of electrons. “A” is the mass number which is also equal to the number of protons + neutrons and is also referred to as the number of nucleons. The number of neutrons is equal to A − Z.
Arrange the following forces (weak force, strong force, gravitational force, and electromagnetic force) in order from weakest to strongest and describe their basic responsibilities.
Question 7
What is an isotope, isotone, isobar, and isomer?
Question 8
What is a characteristic X-ray?
Question 9
What is an Auger electron?
The weakest is the gravitational force which is the force that attracts masses, but on the scale of the atomic world it is negligible. Next, the weak force is responsible for nuclear decay. The electromagnetic force exists between all particles which have an electric charge. The strong force is responsible for binding nuclei and exists over a very short range.
Answer 7
An element is defined by its atomic number Z, which represents the number of protons, and gives the element its chemical properties. The same element can have differing numbers of neutrons but the same number of protons, these are referred to as isotopes. An isotone is the opposite, they are nuclei with the same number of neutrons, but different numbers of protons. Isobars are nuclei that have the same number of nucleons (eg, seven protons and eight neutrons, or six protons and nine neutrons), that is the same atomic number. A helpful guide is istoPes have equal number of Protons, isotoNes have equal Neutrons, and isobArs have same atomic weight (A). An isomer is the same nucleus (the same number of protons, neutrons, and the same atomic number) but is an excited, usually unstable state of the nucleus.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree