Fig. 25.1
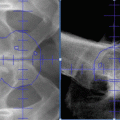
Ga-68 PSMA PET/CT revealed a left parailiac lymph node (LN) of 16 × 19 mm (SUVmax: 5.85)
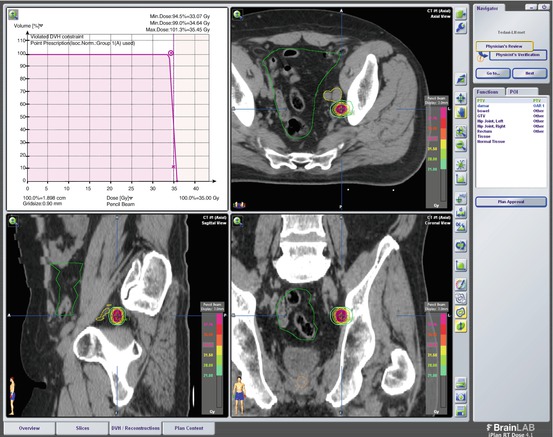
Fig. 25.2
SBRT Plan of Case 1: 35 Gy (7 Gy/day in 5 fractions) stereotactic body radiotherapy (SBRT) was administered to the left parailiac LN via Novalis
25.2.2 Case 2
A 59-year-old male patient with a PSA level of 38 ng/mL was diagnosed with prostate cancer in 2007. His transrectal biopsy of prostate gland revealed an adenocarcinoma with a GS of 3 + 3 = 6 with perineural invasion. His stage was cT3bN0M0. After 9 months of ADT, his PSA decreased to 0.19 ng/mL. Between April 2008 and May 2008, 70 Gy of 3D conformal RT was administered in 35 fractions. ADT was completed to 2 years. In October 2014, his PSA level increased to 4.3 ng/mL, and Ga-68 PSMA PET/CT revealed a pathologic right parailiac lymph node (LN) (SUVmax: 10.5) (Fig. 25.3). Between March 2015 and April 2015, 30 Gy in 5 fractions SBRT was administered to the right external iliac LN via Novalis (Fig. 25.4). In his last follow-up, his PSA level was 0.3 ng/mL on January 2017 with no evidence of disease. We observed no acute or late toxicity.
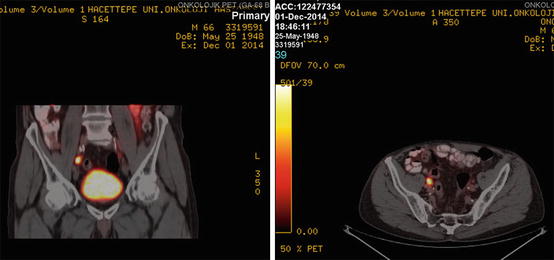
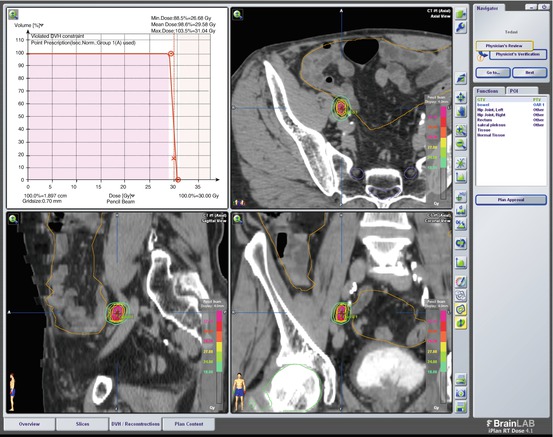

Fig. 25.3
Ga-68 PSMA PET/CT revealed a pathologic right parailiac lymph node (LN) (SUVmax: 10.5)

Fig. 25.4
SBRT Plan of Case 2: 30 Gy in 5 fractions SBRT was administered to the right external iliac LN via Novalis
25.2.3 Case 3
A 76-year-old male was diagnosed with prostate cancer after his PSA level was found to be 10 ng/mL in 2011. Prostatic biopsy revealed an adenocarcinoma with a GS of 4 + 3 = 7. His stage was cT3bN0M0. After 3 months of ADT, 74 Gy IMRT was administered to the prostate and proximal seminal vesicles between June 2011 and August 2011.
In February 2016, his PSA level increased to 5 ng/mL, and Ga-68 PSMA PET/CT revealed recurrence in left external iliac LN (SUVmax: 5.4) (Fig. 25.5). On February 2016, he received 35 Gy in five fractions SBRT to the recurrent area (Fig. 25.6). While prostate cancer-free, he was succumbed to brain metastasis of small cell lung cancer in November 2016.
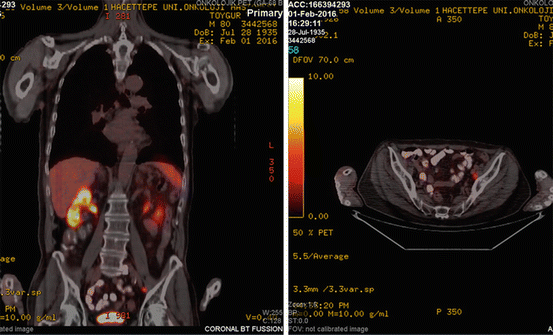
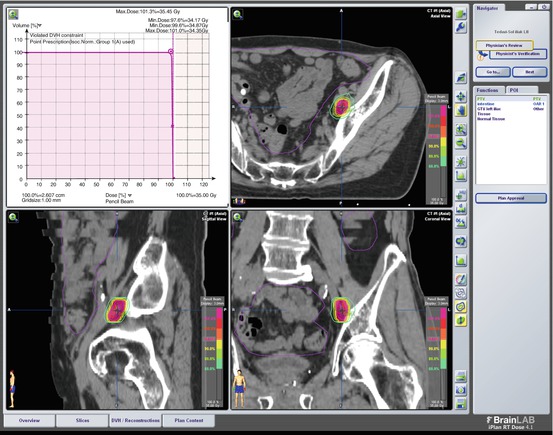

Fig. 25.5
Ga-68 PSMA PET/CT revealed recurrence in left external iliac LN (SUVmax: 5.4)

Fig. 25.6
SBRT Plan of Case 3: 35 Gy in five fractions SBRT delivered to the recurrent area
25.3 Radiolabelled Targetted Therapy with Radium-223 Dichloride
Radium-223 dichloride , an alpha-particle emitting radiotherapeutic drug , acts mimicking calcium at bone metastases with increased bone turnover in order to form complexes with hydroxyapatite. The Food and Drug Administration (FDA) has first approved radium-223 dichloride treatment, provided by Bayer HealthCare Pharmaceuticals Inc. (Xofigo Injection), on May 15, 2013 for the castration-resistant prostate cancer patients who have symptomatic bone metastases without any visceral metastasis. FDA approval was due to the randomized, double-blind, placebo-controlled trial randomly enrolling 541 patients to receive intravenous 50 kBq/kg radium-223 dichloride (1.35 μCi/kg) per month prescribed for six cycles plus best standard of care (local external radiotherapy, corticosteroids, antiandrogens, estrogens, estramustine, or ketoconazole) versus 268 patients to receive a placebo plus best standard of care. The interim analysis demonstrated statistically significant overall survival improvement with radium-223 dichloride (14 vs 11.2 months; hazard ratio: 0.70; p = 0.00185) in addition to a delay benefit in time-to-first symptomatic skeletal event [21–25].
Radium-223 dichloride is recommended to be prescribed at a dose of 50 kBq/kg by a relatively slow intravenous injection approximately over 1 min administered every 4 weeks to a total of six doses [21–25]. The intravenous access line or cannula needs to be flushed with isotonic saline before and after injection. The product radioactivity concentration (1000 kBq/mL; 27 μCi/mL) at the reference date (single-use vials containing 6 mL of solution in market) is important in dose calculation as well as decay correction factor for physical decay of radium-223. Typical activity of a treatment is below 8000 kBq with low external radiation exposure due to handling of patient doses. As radium-223 primarily emits 95.3% of its energy as alpha-particles, fractions emitted as beta particles and gamma radiation are 3.6% and 1.1%, respectively. The gamma radiation and its daughters allow for the accurate radioactivity measurement of radium-223 dichloride as well as the detection of contamination.
The major organs with highest expected absorbed radiation doses are bone (mainly osteogenic cells), red marrow, and upper & lower large intestine walls [21]. Therefore, the clinician should be aware of most common (≥10%) adverse reactions of nausea, vomiting, diarrhea, and peripheral edema, in addition to anemia, neutropenia, lymphocytopenia, leukopenia, and thrombocytopenia; while risk of bone marrow failure or continuing pancytopenia risks are around 2%. Hematologic evaluation at baseline and prior to every dose of radium-223 dichloride must be performed; ensuring mandatory counts of absolute neutrophil ≥1.5 × 109/L, platelet ≥100 × 109/L and hemoglobin ≥10 g/dL at first injection, and absolute neutrophil ≥1 × 109/L, the platelet ≥50 × 109/L at subsequent administrations [22–25]. Though no inadvertent overdosing has been reported during clinical trials, it is necessary to know that there is no specific antidote. If there is an unfortunate inadvertent overdosing happens, medical counter measures such as aluminum hydroxide, barium sulfate, calcium carbonate, calcium gluconate, calcium phosphate, or sodium alginate can be considered in addition to general supportive measures [21–24].
Sartor et al. documented the randomized phase 3, double-blind, ALSYMPCA trial , which enrolled patients to receive either six intravenous injections of 50 kBq/kg radium-223 (614 patients) or matching placebo (307 patients) [24]. Symptomatic skeletal events happened in 33% of radium-223 group and 38% of placebo group with longer time to first symptomatic event with radium-223 (median: 15.6 vs 9.8 months; hazard ratio: 0.66; p = 0.00037). The requirement of external radiotherapy for bone pain and spinal cord compression decreased with radium-223 in comparison to placebo; however, radium-223 did not significantly reduce symptomatic pathological bone fracture risks or tumor-related orthopedic surgical intervention [25]. Therefore, radium-223 should be considered one of viable options for symptomatic bone metastases in case of castration-resistant prostate cancer patients.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree