Acknowledgments
The authors report no relevant disclosures or conflicts of interest. Dr. Troen is supported by grants from the National Institutes of Health (K07 AG060266), Veterans Affairs (BX004369), and the Indian Trail Foundation. Dr. Seldeen is supported by grants from Veteran Affairs (RX003396). Illustrations by Sidra Aslam.
Introduction
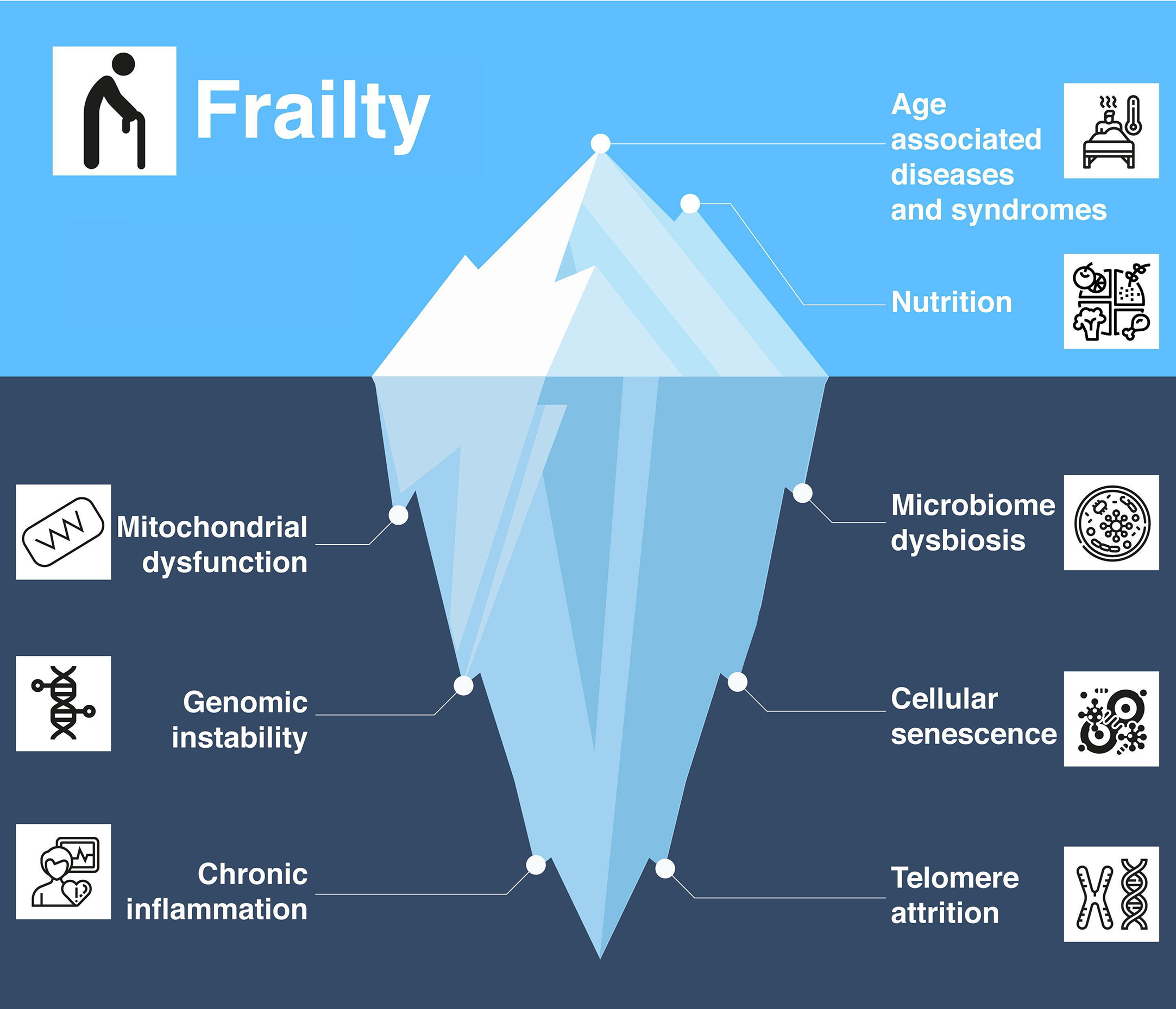
Individuals 65 years of age and older represent the fastest-growing demographic worldwide—accounting for 703 million individuals in 2019 and expected to reach 1.5 billion by 2050 . This rectangularization of the population age pyramid presents significant societal challenges, including the daunting task of addressing age-associated health declines. Of particular concern for older adults is the condition of frailty, defined as a “biologic syndrome of decreased reserve and resistance to stressors, resulting from cumulative declines across multiple physiologic systems, and causing vulnerability to adverse outcomes” . Frailty incidence increases with age, and in the absence of intervention, can be marked by significant functional impairments ( Fig. 1 ) . Frail individuals also have higher rates of disability, institutionalization, and mortality . Therefore, characterizing frailty can provide critical insights that allow for targeted and individually tailored treatment strategies to maintain healthspan, improve quality of life, and reduce healthcare system costs . In this chapter, we examine whether frailty represents the end of an osteosarcopenia continuum resulting in markedly diminished muscle mass and strength, fractures, and impaired functional capacity and quality of life. We further discuss common mechanisms that may lay the foundation for therapeutic strategies.
History of frailty detection and assessment
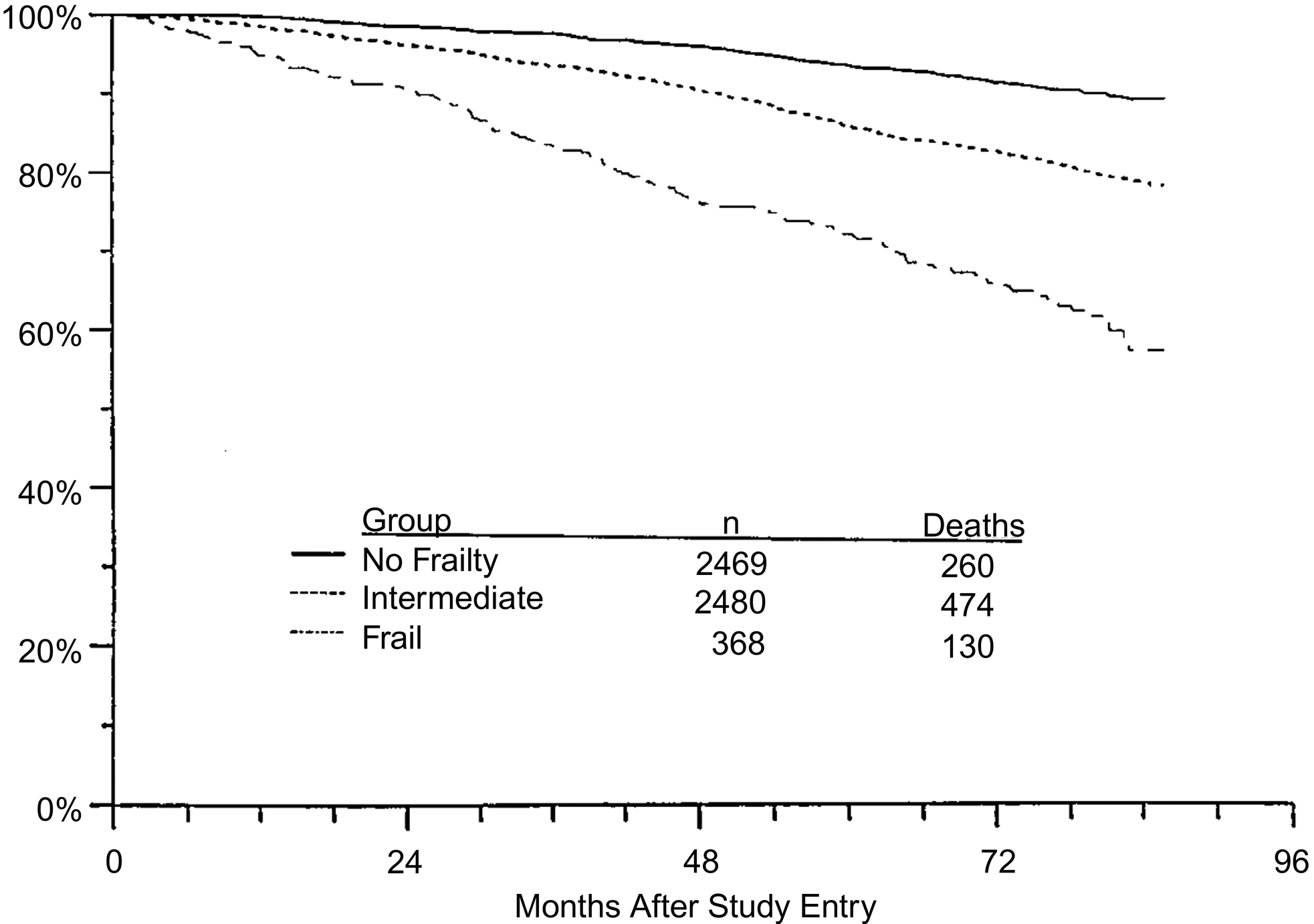
Frailty has often been considered easy to identify, yet difficult to define. Early characterization of frailty recognized core elements, including a continuum of functional decline, failure to thrive, and vulnerability to challenges . Efforts to explore the underlying science of frailty took an important step forward in 2001 with the introduction of the physical frailty phenotype by Fried et al. . For the first time, measurable components were established, which included five factors: unintentional weight loss—10 pounds in the past year, self-reported exhaustion, weakness—lowest quintile of grip strength, slow walking speed, and low physical activity . The presence of 3 of 5 of the factors indicated frailty, and 1 or 2 factors represented prefrailty. In this landmark study involving over 5000 community-dwelling older adults from the Cardiovascular Health Study, frailty was more prevalent with increasing age, in women, and in minority populations . Further, the authors linked frailty status with an increased risk of mortality ( Fig. 2 ). The Fried physical frailty scale has since been used in multiple other studies, and others have linked frailty to a variety of outcomes such as loss of independence, increased disability, and greater morbidity and mortality .
Around this same time, an alternative strategy for the determination of frailty was introduced based upon a deficit accumulation model, whereby a wide range of parameters are taken into account that range from the ability to perform daily activities, coordination and gait, mental components, physiological problems and history, and the presence of medical morbidities . In total, the instrument termed the clinical frailty index, applied 70 parameters in the initial use and identified that frailty index scores increased with age, the severity of impairment when comparing well and unwell groups and that survival probability could be estimated . Subsequently, the 70 parameters were pared down to 36 parameters in an effort to improve clinical utility . Similar to the Fried physical frailty scale, the frailty index has laid a foundation for future research, and together these frailty tools characterize two major schools of thought for scientific frailty assessment that includes dozens of tools . Furthermore, these concepts are echoed in preclinical mouse and rat models of frailty that can accelerate the translation of potential healthspan enhancing interventions .
Frailty detection in clinical situations
Despite the success of frailty assessment for elucidating the biological underpinnings of age-associated declines, the practical clinical utility was questionable due to both the time to administer the tests as well as needing patients to participate in physical performance challenges. In an effort to circumvent these difficulties, a new generation of frailty assessment tools has featured survey questions to replace physical tests. One such instrument is the FRAIL scale that transforms the Fried criteria (fatigue, resistance, ambulation, illnesses, and loss of weight) into 5 self-reported measures ( Table 1 ) . Other clinically useful frailty scales have been developed that feature a greater range of domains. Such scales include the Frailty Trait Scale that explores seven dimensions (energy balance-nutrition, physical activity, nervous system, vascular system, strength, endurance, and gait speed) , the Tilburg Frailty index that encompasses 15 questions within 3 domains (physical, psychological and social) , and the Groningen Frailty indicator that measures the loss of function through 15 questions in 4 domains (physical, cognitive, social and psychological) . Additional tools include the 2‑min Gill frailty instrument, the Gerontopole frailty screening tool, and the PRISMA questionnaire , as well as the frailty index for elders (FIFE) . The proliferation of frailty instruments reflects the effort to implement clinically efficient screens in multiple settings. Furthermore, some individual measures such as gait speed or grip strength may inform future frailty susceptibility . In particular, usual walking speed was found to be the most highly correlated across multiple fitness tests with frailty status .

Alternatively, some clinicians opt to conduct a comprehensive geriatric assessment (CGA), which often takes approximately 2 h per patient to complete . The core domains of CGA are functional status, mobility, gait speed, cognition, mood, and emotional status, nutritional status, comorbidities and polypharmacy, geriatric syndromes (e.g., falls, dementia, delirium, urinary incontinence, dentition, visual, or hearing impairments), goals of care, and advanced care planning. A patient’s social and environmental situation is also evaluated, focusing on the social interactions network, social support needs and resources, financial concerns, and environmental adequacy and safety. CGA produces a holistic inventory of health problems, which can then serve to develop an individualized geriatric intervention plan. In many settings, the CGA relies on a core team consisting of a physician (usually a geriatrician), a nurse, and a social worker. When appropriate, specialists in several other disciplines either take part in the basic assessment or act as consultants with an “extended” team of physical and occupational therapists, nutritionists, pharmacists, psychiatrists, psychologists, dentists, audiologists, podiatrists, and opticians. Program setting, goals of assessment, availability of resources, and caseload influence the size of the core and extended team. At present, CGA programs are moving toward a “virtual team” concept in which members are included as needed, assessments are conducted at different locations on different days, and team communication is completed via telephone or electronically. The strategy has emerged as the gold standard approach for ensuring optimal outcomes in older populations. Still, due to the clinician’s time investment and the inability to provide CGA to all patients, uncertainty remains regarding who would benefit most . Consequently, frailty instruments are increasingly being looked at as a possible screening tool in advance of a CGA . In particular, the rapid geriatric assessment, which takes approximately 4 min and includes the FRAIL scale ( Table 1 ), and screens for sarcopenia, nutrition, and cognition , can identify those who would benefit from a CGA and/or a geriatrician.
Frailty demographics and prognosis
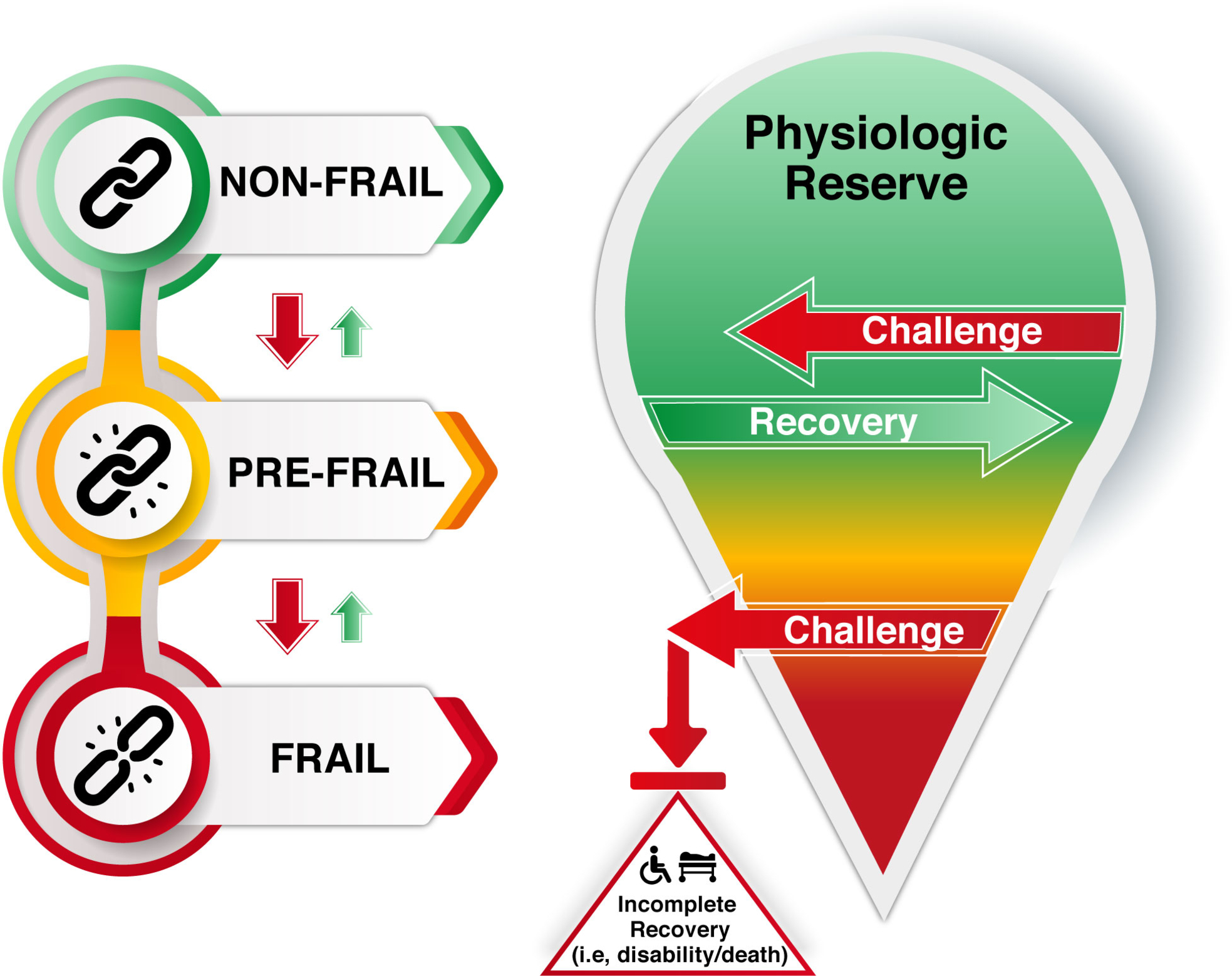
Frailty is defined as a clinically recognizable state of increased vulnerability resulting from aging-associated declines in reserve and function across multiple physiologic systems that compromise the ability to cope with everyday or acute stressors . The predominant risk factor for frailty is age ( Fig. 3 ). Although the prevalence is lower at early old age (approximately 7%–10% of those around the age of 65 years ), this rapidly increases and can affect 25%–50% of those 85 years of age and older .
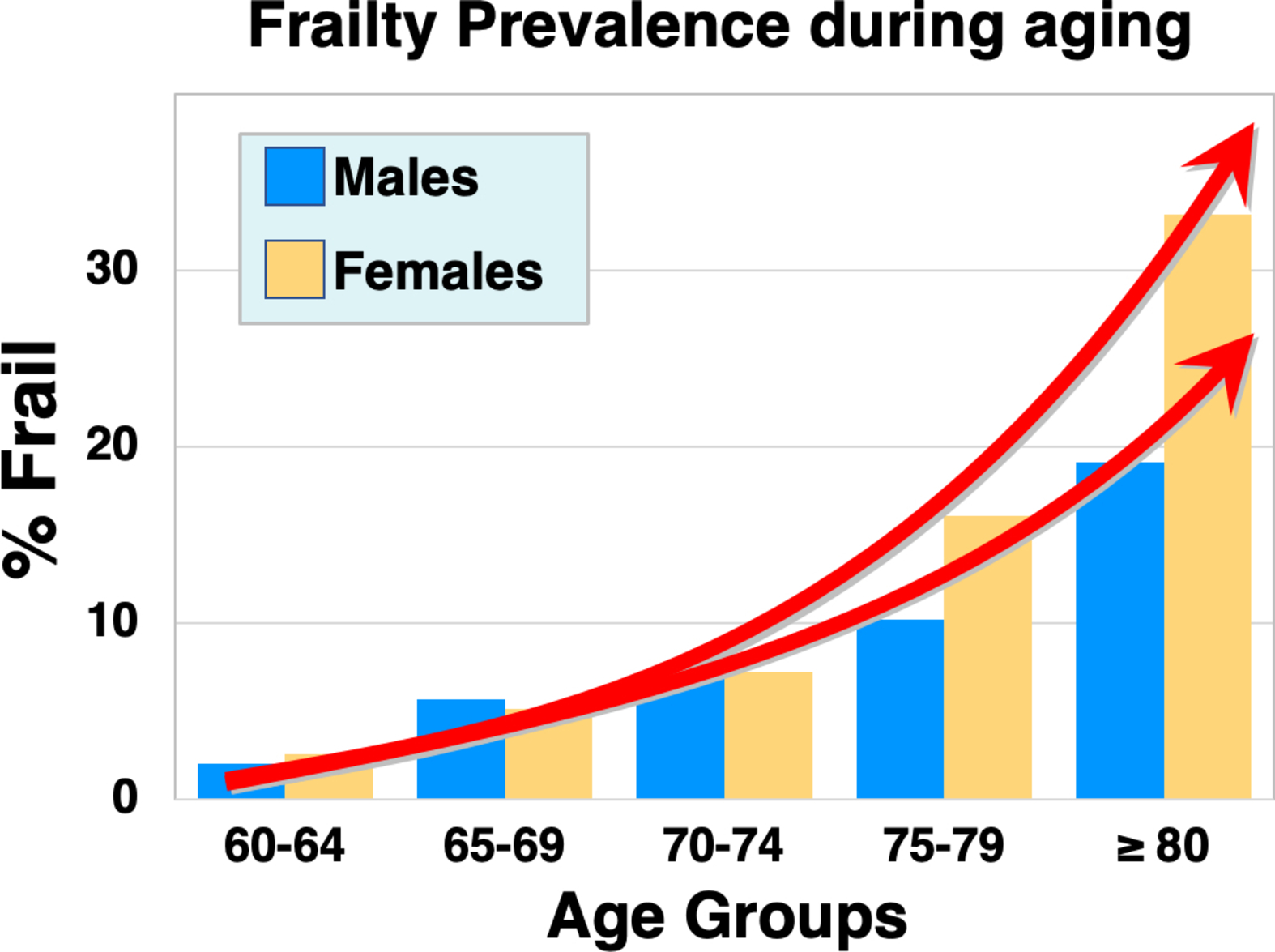
Women are also at higher risk for frailty , but paradoxically can survive with frailty for more extended periods . Frailty also is more pronounced in minority groups, including African Americans who can exhibit rates 4 times higher than Caucasians . Other risk factors for frailty include smoking, obesity, loneliness , socio-economic status, malnourishment , and importantly, inactivity . Inactivity, in particular, is a major risk factor for many factors that contribute to frailty, including obesity and osteosarcopenia , and may be a key trigger that initiates and accelerates the cycle of functional decline that culminates in frailty .
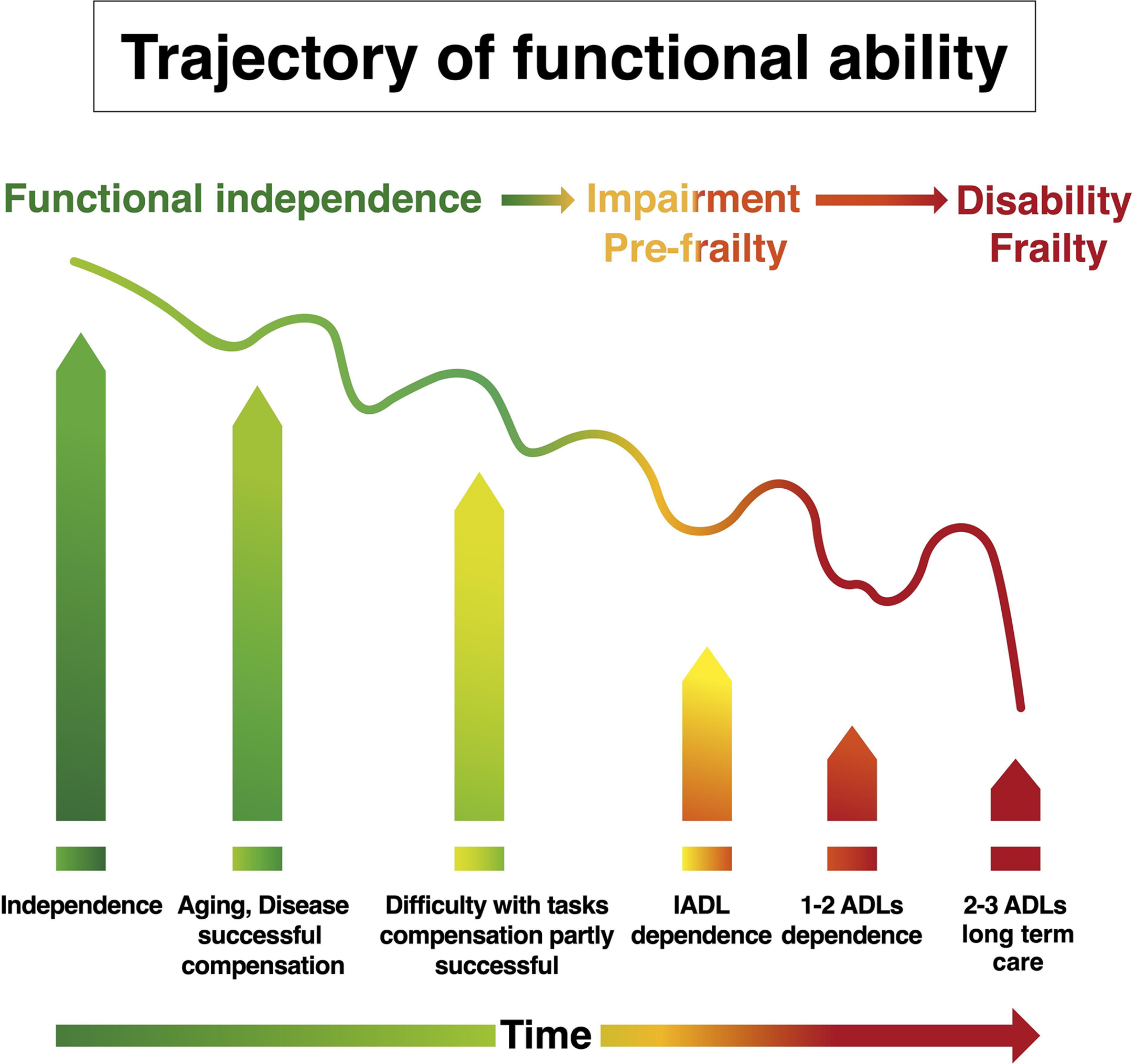
A frailty diagnosis carries a prognosis for adverse events and a higher risk of all‑cause mortality. This relationship has been identified across multiple studies and frailty assessment tools . Specifically, frailty increases mortality risk by over 150%, and even prefrailty increases the risk by 60%–80% . Notably, frailty status was predictive of mortality in large patient cohorts, including nearly 500,000 UK Biobank participants and 5000 participants in the National Health and Nutrition Examination Survey . Very importantly, quality of life is diminished in frail older adults . This may be due to approximately a twofold increase in physical limitations , as well as a twofold increased risk for impairment of activities of daily living . As might be expected with the individual challenges of frailty, there is also a greater likelihood of hospitalization and need for institutional care . Therefore, frailty in older adults places a significant burden on healthcare systems, as also exemplified by increases in expenditures of 50%–100% and utilization of services . The progression of frailty is best characterized as a collection of health challenges, with failure to recover to previous baselines and resultant progressive declines, particularly concerning instrumental activities of daily living (IADLs: shopping, housework, finances, food preparation, medications, transportation) and subsequently to basic activities of daily living (ADLs: dressing, eating, ambulating, toileting, hygiene) ( Fig. 4 ). This phenomenon has been reported in the literature by two studies that examined frailty status longitudinally and identified fluidity in the stages (transitioning bi-directionally between nonfrail, prefrail, and frail status), but with a trend toward increasing disability and frailty .
In addition, frailty status has been shown to be very important as a prognostic indicator for adverse events following surgery in multiple settings . These studies demonstrate the potential of incorporating frailty determination to inform critical decision-making and risk assessment. In fact, the utility continues to expand and has also recently been used to identify at-risk patients exhibiting COVID-19 infection .
Underlying pathophysiology of frailty
Frailty is considered as one of the many syndromes of aging. Inherent in the aging process are systematic declines at both the cellular and tissue levels . Hallmarks include genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, deregulated nutrient sensing, mitochondrial dysfunction, and cellular senescence . These factors play a role in the onset of frailty, but of particular interest are chronic inflammation, a dysregulated gut microbiome, and underlying miRNA signaling in mediating age-related declines. Chronic inflammation, often labeled “inflammaging,” refers to the lingering and frequently subclinical elevation of serum and tissue cytokines including interleukin‑6 (IL‑6), IL‑8, IL‑1, tumor necrosis factor (TNF) alpha, and C‑reactive protein (CRP) . The combination of obesity and sarcopenia can be a dual path to frailty . Notably, adipose tissue produces more inflammatory cytokines that exert a catabolic effect, reducing muscle mass and quality, thereby leading to greater functional decline and frailty . One potential driver of chronic inflammation stems from deleterious alterations in the gut microbiome during aging , which increase vulnerability to malnutrition, inactivity, disease, or response to drug treatments . This in turn diminishes anabolic responses and can reduce muscle mass, and again lead to both sarcopenia and frailty .
Frailty is often thought of as a decline of resilience or the physiological “bounce back” from an acute stressor. Although the microbiome contributes to resilience , epigenetic changes are another key factor that can amplify the deleterious impacts of stressors . Serum microRNA profiles are salient indicators and drivers of epigenetic changes. MicroRNAs are 18–22 base pair fragments of noncoding RNA that can silence messenger RNA expression as well as block gene expression via heterochromatin formation . Specific microRNAs have been implicated in both sarcopenia and other muscle wasting diseases . MicroRNAs are also being investigated as potential biomarkers for frailty , and may well underlie many of the pathologic mechanisms of frailty . Together, the encompassing hallmarks of aging that include chronic inflammation, microbiome dysbiosis, and altered microRNA signaling appear to represent long-term factors leading to the presentation of geriatric syndromes in late life such as frailty ( Fig. 5 ).