Rapid MW protocol logos™ (1 h 39 min)
Reagent
Time
Phase
T °C
Pressure (mBar)
Formalin 10 % NBF
25′
1 = 10′
50°
2 = 15′
Ethanol 60°
2′
Ethanol 99°
3′
Ethanol 99°
16′
1 = 8′
65°
2 = 8′
Isopropyl alcohol
16′
1 = 8′
68°
2 = 8′
Vaporization
1′ 30″
600
Paraffin
35′ 30″
1 = 30″
66°
995
2 = 15′
66°
400
3 = 15′
70°
300
4 = 5′
65°
200
Total: 1 h 39′
4 Tumor Specimens
40 consecutive cases of T1c-T2 surgically treated breast carcinomas (age range 41–67; 39 F,1 M) were evaluated. The fresh resected specimens were received from the operating room (cold ischemia time 10–15 min) and immediately dissected by a pathologist. This series included 34 invasive ductal/NST carcinomas and 4 lobular, 1 tubular, and 1 mucinous carcinomas. All of them were primary tumors, without any previous treatment. At our institution, most patients with either triple negative or HER2 positive tumor are treated with neoadjuvant therapies; therefore, these types of tumors are underrepresented in this series. Two or more fragments no more thicker than 1 mm were obtained from the tumor mass and immediately processed with the protocol outlined in Table 1 in a Logos MW processor (Milestone Serisole Italy). This protocol includes 25′ fixation time in NBF reaching a 50 °C plateau after 10′ and is followed by a 60′ cycle with dehydration in ethanol, clearing with isopropyl alcohol and embedding in paraffin. The main part of the tumor was left in fixative for 24 h and processed the following day with a routine 9 h protocol in a Leica ASP300 instrument. Written informed consent for the procedure was formally obtained from all the patients.
5 Immunostaining
HER2 evaluation was performed on 3 micron sections from cell lines paraffin blocks, bioptic fragments, and routine breast specimens with manual Herceptest™ kit K5204 (Dako) and with HER2 Pathway™ on a Benchmark Ventana Ultra autostainer. In each case, rapid biopsy and routine block sections were immunostained in the same run and with the same standard protocol (HIER, primary ab, detection system). All the samples were also immunostained for ER (clone 6F11, dil 1/200 Novocastra), PR (clone 312, dil Novocastra), and Ki67 (clone MIB1, dil 1/600, Dako) with Refine (Novocastra) as DAB-based polymer detection system in a Bond III automated immunostainer (Leica-Microsystems);
6 FISH
HER2 FISH analysis was performed in eight cases defined as score 2 plus by immunohistochemistry, in both biopsy and surgical sample, with the PathVysion™ HER2 DNA Probe Kit (Abbott Molecular) according to the manufacturer’s instructions. Slides were analyzed using NIKON 90i fluorescence microscope with both a single pass (green and orange) and a triple-pass filter band (DAPI/green/orange); images were captured by Genikon software (Nikon). A total of 100 neoplastic nuclei were observed per each sample.
7 Slides Evaluation
Immunostained slides were evaluated independently by two pathologists (C.D. and I.S) with experience in breast pathology, without knowledge of the pairing (biopsy vs surgical) of the samples. The updated ASCO-CAP guidelines were utilized for HER2 scoring.
8 Results
All the shortly fixed and MW-processed biopsies showed a good morphology on H&E stained sections, with excellent nuclear details and without any artifact (Fig. 1a, b).
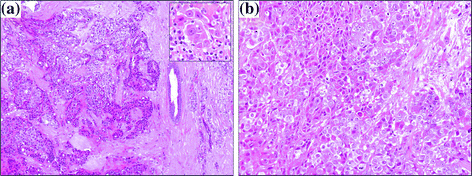

Fig. 1
H&E staining of breast carcinoma rapid biopsy sections demonstrating good morphological quality (a original magnification 100x; insert 400x; b original magnification 200x)
9 Cell Lines
Intense and complete membranous staining for HER2 was detected in the majority of SKBR3 cells, whereas weak, focal, and incomplete staining was present in a limited percentage of MCF7 and T47D cells, irrespective of the fixation time (Fig. 2). In MCF7 and T47D cell lines, a similar percentage of cells with the same intensity, stained for estrogen and progesterone receptors with all the fixation times (30′, 1 h, 6 h, 24 h); SKBR3 cell line, as expected, was negative for ER and PR. A very similar percentage of cells was immunostained at all fixation times with Ki67 in each of the three cell lines.
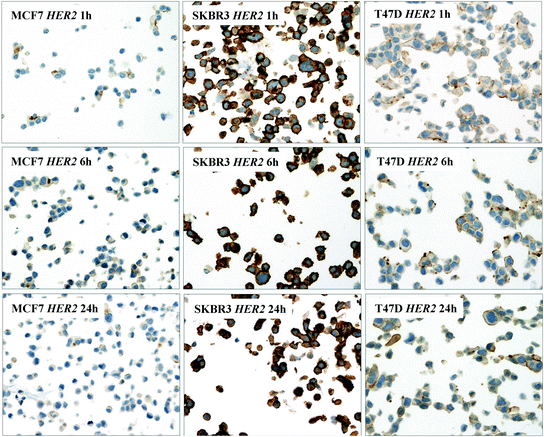

Fig. 2
Breast carcinoma cell lines (MCF7, SKBR3, and T47D) immunostained with HER2—Herceptest™—after different fixation times (1, 6, 24 h) and rapid MW processing, displaying similar staining intensity (original magnification 200x)
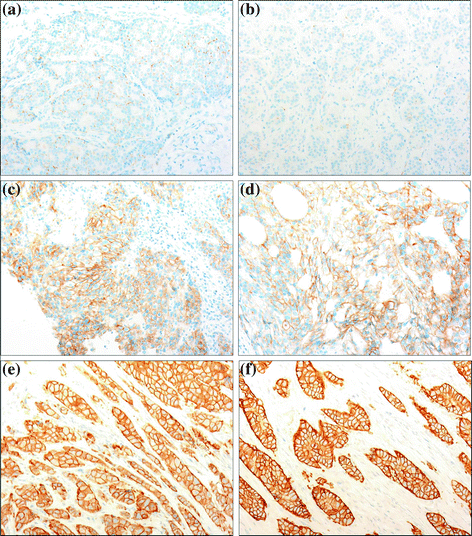
10 Immunohistochemical Results in Rapid Biopsies and Surgical Specimens
The quality of immunohistochemical staining was equivalent in biopsies and paired surgical samples (Fig. 3a–f). No differences were detected in HER2 score between rapid and routine samples, utilizing the Herceptest™ and the HER2 Pathway™ kit. (Table 2) with 3 minor exception: 1 case 1+ in biopsy and 2+ in surgical sample (not amplified by FISH), 1 case 2+ in biopsy and 1+ in surgical sample (not amplified by FISH), and the third case score 0 on biopsy and score 1+ in surgical sample. Some minor scoring differences were observed in some cases, in paired biopsy and surgical sample, also with Herceptest™ versus HER2 Pathway™ kit. Four cases scored 1 plus with the Herceptest™, but had a score 0 with HER2 Pathway™ kit. Normal breast tissue present in biopsies was always negative with HER2 Pathway™ kit; incomplete faint membrane staining of normal tissue was observed in some of the breast biopsies with the Herceptest™. In these cases, a similar staining intensity was present also in the paired surgical sample. For estrogen and progesterone receptors, no statistically significant differences in the percentage of stained cells and in the intensity of the reaction was observed in rapid biopsies compared with the routine surgical specimen, both by visual evaluation and by Aperio Digital Image software analysis, with no more than 1 point difference in the Allred score in paired samples (data not shown).