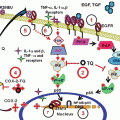
Fig. 1
Chemical structure of potential anticancer factors from sesame seed and proposed underlying mechanisms for the suppression of polyp formation in Min mice by Sesamol. Ferulic acid, sesamin, sesamolin, γ-tocopherol, and sesamol are the major sesame seed constituents that possess the potential anticancer effects. Indeed, in our experiments, sesamol suppressed intestinal polyp formation in Min mice. Interestingly, expression levels of PGE2 receptor subtypes EP1 and EP2 were reduced in the polyp parts with sesamol administration. Furthermore, sesamol inhibited transcriptional activity of COX-2 promoter in colon cancer cells partly through the downregulation of NOX1 expression level. These results suggest that NADPH oxidase is an important target and sesamol could be a promising candidate for colorectal cancer chemoprevention
3 Bioactivities and Anticancer Effects of Sesame Seed Constituents
3.1 Ferulic Acid
Ferulic acid has been reported to improve glucose tolerance and lipid metabolism (El-Seedi et al. 2012; Ardiansyah et al. 2006). Recently, Wang et al. reported that ferulic acid administration alleviated high-fat and high-fructose diet-induced metabolic syndrome parameters, such as obesity, hyperglycemia, hyperlipidemia, and insulin resistance (Wang et al. 2015). Several studies have shown that ferulic acid acts as a potent antioxidant by scavenging free radicals in rats (Sudheer et al. 2005). Antioxidants are considered potential inhibitors of cancer by protecting critical cellular molecules from oxidative damages. Indeed, ferulic acid suppresses chemically induced colon (Kawabata et al. 2000), skin (Asanoma et al. 1994), and lung carcinogenesis (Lesca 1983) in animals.
3.2 Sesamin
Sesamin is a furofuran-type lignan in sesame seed oil, which has potential benefits for health promotion (Pathak et al. 2014). Studies have shown that sesamin plays a significant role in lipid metabolism through the downregulation of lipogenic enzymes (Peñalvo et al. 2006), sterol regulatory element binding protein-1 (SREBP-1), acetyl-CoA carboxylase, and fatty acid synthase (Ide et al. 2001) in animals. In addition, sesamin inhibits cholesterol absorption and biosynthesis in humans (Hirose et al. 1991; Hirata et al. 1996). These bioactivities may be beneficial for preventing the obesity and atherosclerosis. Sesamin showed anti-inflammatory activities through the inhibition of Δ5 desaturase, a key enzyme in arachidonic acid biosynthesis that leads to a lower level of pro-inflammatory mediators (Shimizu et al. 1991; Chavali et al. 1998). As inflammation plays an important role in carcinogenesis, sesamin could be a good candidate cancer preventive agent. Indeed, Hirose et al. reported that the administration of 0.2 % sesamin significantly reduced the number of 7,12-dimethylbenz[a]anthracene (DMBA)-induced palpable mammary cancers compared with control animals (Hirose et al. 1992).
3.3 Sesamolin
Sesamolin has been demonstrated to increase the activity and mRNA level of various enzymes involved in hepatic fatty acid oxidation in rats (Lim et al. 2007). Sesamolin is also known as an antioxidant and has been shown to have a protective effect in neuronal cells against hypoxic and lipopolysaccharide (LPS)-induced toxicity or excitotoxicity in vitro and in vivo (Hou et al. 2003a, b; Cheng et al. 2006). In addition, Miyahara et al. reported that sesamolin inhibited proliferation and induced apoptosis in human lymphoid leukemia Molt 4B cells (Miyahara et al. 2001).
3.4 γ-Tocopherol
γ-Tocopherol, the most abundant tocopherol in sesame seed oil, showed a very broad range of medicinal properties (including anticancer, antidiabetic, anti-inflammatory, and anti-oxidative effects) not only in preclinical studies but also in human intervention studies (Jiang 2014). In a preclinical model, γ-tocopherol administration attenuated dextran sulfate sodium (DSS)-induced colitis and azoxymethane (AOM)/DSS-induced colon tumorigenesis (Jiang et al. 2013), partly through the suppression of eicosanoids [prostaglandin (PG) E2 and leukotriene B4] production (Ju et al. 2009). In addition, Takahashi et al. demonstrated that the supplementation of γ-tocopherol suppressed prostate tumor progression with the induction of apoptosis through caspase activation in the transgenic rats for adenocarcinoma of prostate (TRAP) model (Takahashi et al. 2009). Thus, γ-tocopherol exhibits strong anti-oxidative and anti-inflammatory effects in many studies, and it could be a good candidate as cancer preventive agent.
3.5 Sesamol
Sesamol is involved in the oil-soluble compartment as oil-soluble lignans. Sesamol possesses antioxidant (Hsu et al. 2006), anti-inflammatory (Chavali et al. 2001), and free radical scavenging activity (Parihar et al. 2006). As neutrophil-derived hydrogen peroxide (H2O2) plays an important role in the progression of inflammatory bowel disease (IBD), a previous report examined the sesamol protective effects on dinitrochlorobenzene (DNCB)-induced mucosal injury in a rat IBD model. Sesamol (100 mg/kg, po) treatment for 7 days significantly decreased the levels of myeloperoxidase, thiobarbituric acid reactive species, and nitrite in the colon tissue (Kondamudi et al. 2013). In a long-term animal experimental study, sesamol treatment at dietary levels up to 2 % for 2 years was shown to decrease the spontaneous development of preneoplastic hepatocytic foci in F344 rats (Hagiwara et al. 1996).
In our experiments, among the four sesame seed constituents (ferulic acid, sesamin, sesamol, and sesamolin), 100 μM sesamol treatment in a human colon cancer cell line, DLD-1 cells, for 48 h suppressed basal cyclooxygenase-2 (COX-2) promoter transcriptional activity as detected by a β-galactosidase reporter gene system (Mutoh et al. 2000; Shimizu et al. 2014). COX-2 is an inducible enzyme to produce PGE2, and both COX-2 expression and PGE2 levels are increased in colon carcinoma tissues compared with that of normal colonic mucosa. Accumulating evidence suggests that COX inhibitors suppress colon carcinogenesis in animal experiments and human trials (Komiya et al. 2013; Mutoh et al. 2006). It has also been reported that COX-2 gene knockout results in the reduction of intestinal polyp development in a model of human familial adenomatous polyposis, Min mice (Oshima et al. 1996). Thus, it is likely that agents that can suppress COX-2 expression at the transcriptional level may also suppress colon carcinogenesis in animal experiments and human trials.
We have shown the suppressive potential of sesamol on intestinal polyp development in Min mice (Shimizu et al. 2014). The administration of 500 ppm sesamol reduced the total number of intestinal polyp development compared with that of the untreated group. When the small intestine is divided into three parts, the proximal, middle, and distal parts, sesamol decreased the number of polyps in the middle part.
In the polyp parts of Min mice, sesamol suppressed COX-2 mRNA expression levels. Sesamol treatment also reduced cytosolic prostaglandin E synthase (cPGES) mRNA expression levels. Moreover, a tendency of suppression in the expression levels of PGE2 receptor subtypes EP1 and EP2 in the polyp parts was observed. We confirmed the effect of sesamol on human EP1 and EP2 mRNA levels in DLD-1 cells and found that sesamol significantly suppressed EP1 and EP2 mRNA levels. PGE2 receptor subtype-knockout mice have demonstrated that EP1, EP2, and EP4 are promotive receptors in colorectal carcinogenesis, and EP3 plays suppressive roles (Mutoh et al. 2006). Unfortunately, there are a few inhibitors for PGE2 receptor subtypes. Thus, the novel potential of sesamol may be worthwhile from the aspect of developing chemopreventive agents.
In addition, we performed a preliminary experiment using intestinal polyp samples of Min mice to examine effects of sesamol on the expression levels of oxidative-related factors. We obtained the data showing a tendency toward the reduction of NOX1 mRNA by sesamol as described in the next section.
4 Possibility of NOX as a Novel Cancer Prevention Target
4.1 Nicotinamide Adenine Dinucleotide Phosphate Oxidase
Cancerous tissue is known to be under oxidative stress conditions. Distinct amounts of ROS are produced as a by-product from the mitochondrial respiratory chain, and a large amount of superoxide anion radical (O2 −) is often generated by cancer cells due to a complex I dysfunction in mitochondria. The initial step in ROS formation is the generation of O2 − and then proceeds to H2O2 and the hydroxyl radical (OH), which causes strong damage to cells (Halliwell 1978). At low ROS concentrations, intracellular signaling is initiated, whereas at high ROS concentrations, oxidative stress is induced. Extensive studies over the years have determined that high ROS conditions contribute to the progression of various human diseases, especially cancer. ROS targets regulate proliferation, including cellular signaling (phosphatases, AP1 and NF-κB) and cell cycle (CDC25, cyclin D, and forkhead proteins; G1, G2, respectively) proteins.
Another enzyme that generates ROS is NOX. Membrane-integrated NOX family oxidases, such as NOX1 and Duox2, are known to produce O2 − or H2O2 (Sumimoto 2008). NOX is a multicomponent enzyme, which is formed of a complex, membrane-associated component (Nox1-5, Duox1, 2, and p22phox), cytosolic components (p47 phox, Noxo1 (homologue of p47 phox), Noxa1 (homologue of p67 phox), and p40 phox), and the small GTP-binding protein Rac (Vulcano et al. 2004; Li and Shah 2001; Muzaffar et al. 2008; Brandes and Schroder 2008).
Correlating with activating mutations in K–ras, NOX1 is overexpressed in human colon cancers (Laurent et al. 2008). Other factors are also elevated in cancer cells and have been suggested to play an important role in carcinogenesis. The function of NOX2 and NOX3 in cancer is not well known. NOX4-generated ROS has been suggested to induce resistance of pancreatic cancer cells to apoptosis (Mochizuki et al. 2006), and its inhibition suppresses melanoma cell growth (Yamamura et al. 2009). On the other hand, Duox1 and Duox2 are remarkably suppressed in lung cancer cells due to the hypermethylation of CpG-rich regions of their promoters (Luxen et al. 2008).
4.2 NOX Inhibitors and Cancer/Carcinogenesis
As described in a previous section, many studies have indicated that the NOX family of genes appears to be required for survival and growth of a subset of human cancer cells. Thus, the NOX family should be a focus of attention in cancer etiology and cancer prevention studies (Kamata 2009). The significance of NOX in carcinogenesis has been shown in experiments using NOX inhibitors. NOX functional inhibitors are classified as (1) small molecule chemical NOX inhibitors and (2) biological inhibitors for NOX, such as neopterin and gp91ds-tat. In this section, we will review and discuss the relationship between NOX inhibitors (apocynin, diphenylene iodonium, vanillin, and methyl-vanillin) and carcinogenesis.
4.2.1 Apocynin (4′-Hydroxy-3′-Methoxyacetophenone; Acetovanillone)
Apocynin is a methoxy-substituted catechol, compound isolated from the traditional medicinal plant P. kurroa, and is structurally related to vanillin. Apocynin is commonly used as an inhibitor of NOX (IC50 = 10 μM) (Stefanska and Pawliczak 2008).
At 300 μM, it is effective in preventing the production of O2 − in human white blood cells and neutrophilic granulocytes. Due to the selectivity of its inhibition, apocynin can be widely used as an NOX inhibitor without interfering in other systems. It has been used in the treatment of arthritis, bowel disease, asthma, atherosclerosis, and familial amyotrophic lateral sclerosis (Stefanska and Pawliczak 2008; Heumüller et al. 2008).
Studies have shown the ability of apocynin to decrease O2 − generation in bovine pulmonary arteries as well as neutrophils and macrophages at 10 μM. In experimental rats, apocynin displayed anti-inflammatory activity and improved endothelial function by reducing oxidative stress. On the other hand, Heumüller et al. insisted that apocynin predominantly acts as an antioxidant in endothelial cells and vascular smooth muscle cells and should not be used as an NOX inhibitor in vascular systems (Heumüller et al. 2008).
Regarding carcinogenesis, Suzuki et al. noted that apocynin possesses chemopreventive potential against prostate cancer, using the TRAP model. The ratio and numbers of carcinomas in prostates were significantly reduced by apocynin treatment, with dose dependence (Suzuki et al. 2013a). The authors also found that apocynin significantly inhibited cell proliferation of the prostate cancer cell line PLS10 via inducing a G1 arrest of the cell cycle in vitro. Surprisingly, apocynin did not affect ROS production but inhibited phosphorylation of Rac1, a component of the NOX complex (Suzuki et al. 2013b).
In the same experiments, the expression and secretion of vascular endothelial growth factor (VEGF), important growth factor for malignant progression, were reduced by apocynin treatment (Suzuki et al. 2013b). In addition, it has been reported that ROS suppress adiponectin production in adipocytes, and treatment of obese mice with anti-oxidative agents improves insulin resistance and restores adiponectin production (Matsuda and Shimomura 2014). We previously discovered that mice with disruptions in adiponectin loci develop an increased number of intestinal tumors compared with wild-type mice (Mutoh et al. 2011). Therefore, we suggest that apocynin could be a direct and indirect chemopreventive reagent for carcinogenesis.
4.2.2 Diphenylene Iodonium
Diphenylene iodonium (DPI) is the most commonly used NOX inhibitor. DPI abstracts an electron from transporter and forms a radical, which inhibit the respective electron transport through a covalent binding step to flavin proteins. DPI is a very effective NOX inhibitor; however, it interferes with many other enzymes, such as nitric oxide synthase, xanthine oxidase, mitochondrial complex I, cytochrome P-450, etc. Therefore, DPI effectiveness could be due to effects other than its specific activity against NOX (Bedard and Krause 2007).
On that assumption, DPI could function in the malignant process. For example, DPI treatment strongly inhibits ROS generation and HIF-1α expression in MDA-MB-231 cells as well as cell migration and invasion. The inhibition of ROS production with DPI attenuates ERK1/2 activation (Liu et al. 2014).
4.2.3 Vanillin and Methyl-Vanillin
As noted above, apocynin is structurally related to vanillin, and apocynin powder has a fragrance similar to vanilla. Based on the structural resemblance, vanilla could function as an NOX inhibitor. Glutathione (GSH), cysteine, ovalbumin, and the coenzyme NADPH were chosen as potential target biomolecules that could be affected by transient free radicals from vanillin and vanillic acid (Castor et al. 2010).
Respiratory burst activity was suppressed by a previous infusion of 2 mM vanillin. Vanillin suppresses the respiratory burst activity of Kupffer cells as assessed in intact liver, which may be associated with the inhibition of macrophage NOX activity (Galgani et al. 2012).
Vanillin and apocynin inhibit cell migration, and both compounds selectively inhibit Akt phosphorylation of HGF signaling without affecting the phosphorylation of Met and Erk. Furthermore, vanillin and apocynin inhibit the enzymatic activity of phosphoinositide 3-kinase (PI3K), as revealed by an in vitro lipid kinase assay, suggesting that the inhibition of PI3K activity was a mechanism underlying the inhibitory effect on cancer cell migration, and the presence of an aldehyde or ketone group in the vanillin structure was important for this inhibition (Lirdprapamongkol et al. 2009).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree