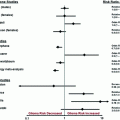
Trial
Vaccine
Type
Phase
Experimental design
N
PFS (mo)
OS (mo)
NCT00612001 [32]
DC
I
Autologous DC + selected glioma antigens
6
9.6
18.1
NCT00846456 [59]
DC
I/II
Autologous DC transfected with GSC mRNA for GBM
7
22.3
24.4
NCT00323115 [60]
DC
II
Autologous DC + tumor lysate for new GBM by intranodal injection
10
9.5
28
NCT00576641 [31]
ICT-107
DC
I
Autologous DC + GSC peptides for new GBM
17
16.9
38.4
NCT00068510 [33]
DCVax-L
DC
I
Autologous DC + tumor lysate for malignant glioma
28
18.1
34.4
NCT00576537 [34]
DC
II
Autologous DC + tumor lysate for malignant glioma
32
8
18
NCT01280552 [61]
ICT-107
DC
IIb
Randomized, placebo-controlled DC + selected peptides for new GBM
81
11.2
18.3
NCT02049489
ICT-121
DC
I
DC Vaccine against CD133 for recurrent GBM
Ongoing
NCT02010606
DC
I
Autologous DC pulsed with allogenicglioma stem cell lysate for new and recurrent GBM
Ongoing
NCT01957956
DC
I
Autologous DC + tumor lysate for new GBM
Ongoing
NCT01808820
DC
I
Autologous DC + tumor lysate vaccine + imiquimod for HGG
Ongoing
NCT00890032
DC
I
GSC mRNA loaded DC after surgery for recurrent GBM
Ongoing
NCT00639639
DC
I
CMV pp65-LAMP mRNA loaded Dcfor new GBM
Ongoing
NCT01204684
DC
II
Autologous DC + tumor lysate + Poly-ICLC for malignant glioma
Ongoing
NCT00045968
DCVax-L
DC
III
Randomized, placebo-controlled DC + tumor lysate for new GBM
Ongoing
NCT00293423 [38]
HSPPC-96
HSP
II
Autologous HSP vaccine for recurrent GBM
41
5
10.5
NCT00905060 [39]
HSPPC-96
HSP
II
Autologous HSP vaccine for new GBM
46
16
23.3
NCT01814813
HSPPC-96
HSP
II
HSP vaccine + bev versus bev alone for recurrent GBM
Ongoing
NCT00643097 [26]
PEP-3
Peptide
II
EGFRvIII vaccine + GM-CSF for new GBM – Part I (ACTIVATe)
18
12.3
20.4
NCT00643097 [26]
PEP-3
Peptide
II
EGFRvIII vaccine + GM-CSF for new GBM- Part 2 (ACT II)
22
15.3
20.5
NCT00458601 [62]
PEP-3
Peptide
II
EGFRvIII vaccine + GM-CSF for new GBM- Part 3 (ACT III)
65
12.3
21.8
NCT02149225
GAPVAC
Peptide
I
Personalized polypeptide vaccine + Poly-ICLC for new GBM
not open
NCT01222221
IMA950
Peptide
I
Multivalent Peptide Vaccine + GM-CSF for new GBM
Ongoing
NCT01250470
ISA-51
Peptide
I
Survivin peptide vaccine for malignant glioma
Ongoing
NCT01854099
PEP-CMV
Peptide
I
CMV Antigen vaccine for new GBM
Withdrawn
NCT01621542
WT2725
Peptide
I
WT peptide vaccine for advanced solid malignancies (including GBM)
Ongoing
NCT01400672
Peptide
I
Allogenic BTIC cell line lysate + imiquimod for DIPG
Ongoing
NCT00069940
Peptide
I
Telomerase peptide vaccine + GM-CSF for sarcomas or GBM
Ongoing
NCT01920191
IMA950
Peptide
I/II
Multivalent peptide vaccine + Poly-ICLC + TMZ for new GBM
Ongoing
NCT02078648
SL-701
Peptide
I/II
Multivalent peptide vaccine + imiquimod for recurrent GBM
Ongoing
NCT01498328
PEP-3
Peptide
II
EGFRvIII vaccine + GM-CSF + BEV versus Placebo + BEV for recurrent GBM (ReACT)
Ongoing
NCT01480479
PEP-3
Peptide
III
EGFRvIII vaccine + GM-CSF versus Placebo for new GBM- Part 3 (ACT IV)
Ongoing
2.1 Peptide Vaccines
Systemic delivery of tumor-specific peptides or cell fragments can be used to educate and activate CTLs through uptake and presentation by dermal APCs. The process of uptake and expression of the antigenic peptides is enhanced by conjugating them to immune stimulants, such as keyhole limpet hemocyanin (KLH), and/or simultaneously administering leukocyte growth factors, such as GM-CSF or IL-2. The key to this approach is delivering peptides that are significantly expressed and highly specific for the tumor. Vaccines can be created by selecting antigenic target(s) and generating synthetic peptides, by extracting specific peptides from tumor lysates, or by nonspecifically delivering tumor cell lysates with a variety of antigenic peptides.
The most investigated target of a specific peptide vaccine in GBM is the tumor variant epidermal growth factor receptor (EGFRvIII). Amplification and overactivation of EGFR is a common mutation seen in GBM [23]. Approximately 30–40 % of GBM patients express an aberrant receptor, EGFRvIII, which remains constitutively active regardless of ligand binding, driving cell activity [24]. EGFRvIII is only expressed by GBM cells, making it an ideal target for vaccine therapy. A peptide vaccine containing a 14 amino acid antigenic sequence for EGFRvIII conjugated to KHL (rindopepimut) has been studied in phase II and III trials for newly diagnosed and recurrent GBM [25]. In the ACTIVATE trial, 18 patients received the vaccine and concurrent GM-CSF for newly diagnosed GBM following surgical resection and standard concurrent temozolomide chemotherapy and conformal radiotherapy (NCT00643097). The median progression-free survival (PFS) in the study was 12.3 months. In the ACT II trial, 22 patients received the vaccine after standard therapy and went on to adjuvant temozolomide therapy after progression (NCT00643097). Median PFS was 15.3 months and overall survival (OS) was 20.5 months [26]. The primary outcome measure in these trials was PFS, which faired favorably against historical control data from disease-matched patients with a median PFS of 6.4 months. These results led to the ACT III study, a phase II trial of the vaccine following surgery and chemoradiotherapy for newly diagnosed GBM, given concurrently with maintenance temozolomide (NCT00458601). The primary outcome for this trial was also PFS, with a median PFS of 12.3 months and a median OS of 21.8 months. A phase III trial of the EGFRvIII vaccine with adjuvant temozolomide versus placebo with temozolomide is currently ongoing (ACT IV; NCT01480479). Additionally, a phase II trial of the vaccine with bevacizumab versus bevacizumab alone for recurrent GBM in adults is ongoing (ReACT; NCT01498328).
In addition to EGFR, a number of other target proteins have been used to develop peptide vaccines for GBM. A Japanese phase II trial of a peptide vaccine targeting the Wilms Tumor (WT1) protein in recurrent gliomas demonstrated a median PFS of 5 months [27]. A phase I trial of a WT vaccine for advanced solid malignancies including GBM is now underway in the United States. Other phase I trials targeting a survivin peptide, CMV antigens, and telomerase are ongoing (see Table 1). The challenge for peptide vaccines with a single target is that their use is limited to patients who express the target. In the case of EGFRvIII, only 30–40 % of patients are eligible to receive the vaccine based on target protein expression. Furthermore, GBM is known to have significant heterogeneity in gene expression from cell to cell within the tumor [28]. Targeting a single protein may lead to eradication of all cells expressing that target, but other cells may survive, resulting in recurrence with selection for tumor that is not recognized by the immune response. This was demonstrated with the EGFRvIII peptide vaccine in the phase II trial [26]. All patients in the trial were histologically proven to have EGFRvIII expression at enrollment; however, of the 11 patients in the trial who underwent biopsy/re-resection at recurrence, 9 of 11 (82 %) had no evidence of EGFRvIII expression in the recurrent tumor [26]. These results suggest that the vaccine was effective in eradicating its target, but facilitated selection of a resistant tumor at recurrence.
To address the concern of limited efficacy with a single antigenic target, a new generation of multivalent peptide vaccines is now in clinical trials for GBM. The peptide vaccine SL-701 is a proprietary multivalent vaccine that has been tested for a mixed group of pediatric high-grade gliomas in a phase I trial with evidence of a positive immunologic response in 81 % of patients [29]. A phase I/II study is now enrolling adult patients with recurrent GBM (NCT02078648). The IMA950 platform contains a proprietary group of 11 synthetic HLA-A2 restricted tumor-associated peptides (TUMAPs) identified by screening a large number of GBM samples [30]. This multivalent peptide vaccine is being studied in patients with newly diagnosed GBM when given in combination with GM-CSF (NCT01222221). A phase I/II study of IMA950 in combination with Poly-ICLC is now recruiting patients as well (NCT01920191). Enrollment in this trial is still limited to HLA-A2 positive patients and it is unclear how many of the target peptides the average individual patient actually expresses.
2.2 Dendritic Cell Vaccines
In contrast to peptide vaccines that rely on endogenous APCs to uptake and display the peptide for T cell stimulation, DC vaccines control this crucial step by ex vivo manipulation. DC vaccines are generated after harvesting a patient’s autologous DCs by plasmapheresis. Cells are stimulated ex vivo and antigenic peptides are introduced by pulsing them with DCs in culture. Activated DCs expressing the antigenic peptides on class I MHC are then reintroduced systemically, facilitating education of naïve T cells. This approach was used for the first cancer vaccine approved by the FDA, developed for the treatment of prostate cancer [16]. In comparison to peptide vaccines that can be given “off the shelf”, this personalized vaccine approach is much more labor intensive and expensive. However, while peptide vaccines require predetermination of the antigenic target to synthesize the immune-stimulatory peptide, DCs can be pulsed with selected peptides or whole tumor lysate, allowing development of a unique, multivalent vaccine targeting the most highly expressed antigens in a particular patient’s tumor.
A number of early phase clinical trials utilizing DC vaccine for GBM have been completed and reported (Table 1). The ICT-107 vaccine utilizes a panel of six HLA-A1/2 restricted peptides known to be highly expressed in glioma stem cells that are pulsed into autologous DCs for generation of the vaccine. In a small phase I study, the vaccine demonstrated highly hopeful results in 17 newly diagnosed GBM patients with a median PFS of 16.9 months and median OS of 38.4 months [31]. This led to a randomized, placebo-controlled phase IIb trial for newly diagnosed GBM (NCT01280552). Although not yet published, the results have been reported, demonstrating that the median OS in 81 vaccine treated patients was only 18.3 months with no significant differences in the treatment and placebo arms. Other DC vaccines with selected glioma-associated antigens have shown similar median OS of approximately 18 months in early phase trials with mixed high-grade gliomas [32].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree