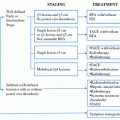
Tumor classification
Definition
Stage
Criteria
T0, N0, M0
Not found
Stage I
T1
T1
One nodule <2.0 cm
Stage II
T2
T2
One nodule 2.0–5.0 cm; 2 or 3 nodules, all <3.0 cm
Stage III
T3
T3
One nodule >5.0 cm; 2 or 3 nodules, at least 1 >3.0 cm
Stage IVa1
T4a
T4a
Four or more nodules, any size
Stage IVa2
T4b
T4b
T2, T3, or T4a plus gross intrahepatic portal or hepatic vein involvement as indicated by CT, MRI, or US
Stage IVB
Any N1, any M1
N1
Regional (portal hepatitis) nodes involved
M1
Metastatic disease, including extrahepatic portal or hepatic vein involvement
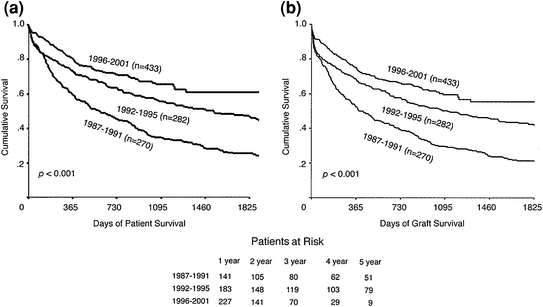
Fig. 1
Patient (A) and graft (B) survival by Kaplan-Meier survival analysis for patients transplanted for hepatocellular carcinoma at different time periods. The lower panel shows the number of patients at risk at each year. (From Yoo H, Patt C, Geschwing J, Thuluvath P, The Outcome of Liver Transplantation in Patients with Hepatolcellular Carcinoma in the United States Between 1987 and 2001: 5-Year Survival Has Improved Significantly with Time. Journal of Clinical Oncology 2003;21(23): 4329-35. Copyright © 2003 American Society of Clinical Oncology with permission.)
Initial allocation in the United States was based on the child-turcotte-pugh score (CTP) which defined 3 categories of disease severity [18]. The highest priority, 2A, was given to patients with CTP ≥10 hospitalized in the intensive care unit. Status 2B was defined as CTP ≥10 not in the ICU or CTP ≥7 with major complications related to cirrhosis such as encephalopathy, spontaneous bacterial peritonitis, variceal hemorrhage, or intractable ascites. Status 3 comprised patients with CTP ≥7 without significant complications of chronic liver disease. Patients with cirrhosis and HCC meeting the criteria defined by Mazzaferro et al. (subsequently known as the Milan criteria) were listed as Status 2B which comprised the vast majority of patients awaiting transplantation and thus waiting time became the prime determinant of organ allocation. This new demand for donor organs already in short supply put additional strain on the CTP-based allocation system. Indeed, up to 45 % of patients on the United Network for Organ Sharing/Organ Procurement and Transplant Network (UNOS/OPTN) waitlist waited at least 2 years for a deceased donor organ [18]. This was particularly a problem in patients with HCC and several studies documented disease progression beyond Milan criteria in 30–50 % of patients within 1 year of listing [19, 20].
4 Introduction of MELD-Based Allocation
Given the dramatically improved results of transplantation for early stage HCC and the shortcomings and the CTP time-based allocation system, many believed that HCC patients were distinctly disadvantaged in organ allocation. Although waiting time was the cornerstone of the tripartite CTP-based allocation system, time spent on the waitlist failed to correlate with risk of death while waiting for a deceased donor organ [21]. This led to efforts to improve deceased donor allocation and find a method to better predict waitlist dropout due to death or tumor progression. In 1998, the United States Health Resources and Services Administration of the Department of Health and Human Services issued the “Final Rule” calling for the development of a new allocation policy based on disease severity and likelihood of dying or becoming non-transplantable while awaiting liver transplantation [22].
In February 2002, the model for end-stage liver disease (MELD) was introduced as the system deceased donor liver allocation in the United States. MELD was originally developed to determine mortality in patients undergoing transjugular intrahepatic portosystemic shunting (TIPS) [23]. Three laboratory values [international normalized ration (INR), bilirubin, and creatinine] determine MELD score and this score determined waitlist ranking. Thus, severity of liver disease became the major determinant of organ allocation. Several studies have validated MELD as an accurate predictor of mortality in patients with end-stage liver disease [24, 25]. Soon after its implementation additions to the waitlist decreased, the rate of transplantation increased, and linear relationship between MELD score and waitlist mortality was retrospectively demonstrated [26]. Although MELD clearly had a beneficial impact on organ allocation in patients with end-stage liver disease, the dilemma arose of how to allocate livers to patients with transplantable HCC and low MELD score before they progressed outside of Milan criteria. Initially, patients with HCC were arbitrarily assigned a score thought to allow for timely transplantation. The goal was to equate risk of progression beyond Stage II disease (based on the American liver tumor study group modified TNM staging system) for HCC patients with risk of death on the waitlist for non-HCC transplant candidates over a similar time period. Patients with Stage I disease were assigned a MELD of 24 which estimates a 15 % chance of progressing beyond Milan criteria in 3 months. Those with Stage II disease were assigned 29 points approximating a 30 % risk of progression within 3 months. Every 3 months thereafter MELD points estimating a 10 % increase in progression beyond Stage II disease were added to the initial allocation score.
Soon after implementation of the new allocation scheme for HCC the number of transplants for HCC increased from 7 % the year preceding MELD to 22 % in the year after and 87 % of patients with HCC were transplanted within 3 months of listing [27, 28]. The incidence of deceased donor liver transplantation for HCC increased from 0.439 transplant/person years pre-MELD to 1.454 transplant/person years post-MELD (p < 0.001) and the mean waiting time for HCC patients decreased from 2.3 to 0.69 years (p < 0.001) [27]. Furthermore, the number of HCC patients who became non-transplantable or died was actually lower than the number of non-cancer patients with similar MELD score who died awaiting an organ [18]. As a result of these and other data, concern grew that HCC patients were given excess priority and the allocation model was changed in 2003 to give patients with Stage I disease 20 points (or an 8 % chance of dying or disease progression in 3 months) and Stage II patients 24 points. This reduced the number of patients transplanted for HCC from 22 to 14 % although it did not significantly change the time on the waitlist or waitlist drop-out in a study of the UNOS database [29]. In an analysis of explant pathology reports, 31 % of patients transplanted for Stage I HCC were found to have no evidence of malignancy in the specimen compared to 9 % of those with Stage II disease [18]. This led to a cessation of MELD allocation priority to those with Stage I tumors. Under current UNOS policy, patients receive an allocation priority MELD of 22 which is increased by an estimated 10 % risk of drop-out every 3 months.
Several studies have retrospectively validated improved survival of patients with HCC within Milan criteria undergoing liver transplantation. In the largest North American single-center experience, Duffy et al. from UCLA present the outcomes of 467 patients who underwent transplantation for HCC from 1984 to 2006 [30]. Recurrence-free survival was significantly higher in patients within Milan criteria compared to those beyond (74 % versus 27 % respectively; p < 0.01). Notably, patients who underwent transplantation after the institution of MELD-based allocation had a 74 % 5-year overall survival versus 47 % of those transplanted prior to implementation of MELD (I = 0.001). In another report from a large American center Onaca and Klintmalm demonstrate similar improvement in outcome following implementation of Milan criteria in selection for transplantation [31]. Five-year overall survival improved from 28.6 % in 1987–1992 to 42.3 % in 1992–1997 likely reflecting general improvements in liver transplantation. These results were inferior to those achieved in non-HCC patients. After 1997, 5-year survival improved to 76 % for HCC patients, similar to the survival in non-malignant indications. Tumor recurrence rates dropped from 52.9 % (1987–1992) and 48.2 % (1992–1997) to 11.4 % (1997–2002). Again, it was demonstrated that MELD-based allocation reduced median wait time for patients with HCC.
5 Pretreatment of Patients Awaiting Transplantation
Despite the improved prioritization for HCC under the MELD-based allocation system there has been an increasing demand of a relatively fixed supply of deceased donor organs. This, in turn, has led to an increased dropout rate from the waitlist and worse overall survival of HCC patients awaiting transplantation [32]. Llovet et al. documented a 25 % drop-out rate in the first 6 months awaiting liver transplantation [20]. In an analysis of the UNOS/OPTN database, Pelletier et al. demonstrated a 12 % 1-year and 20 % 3-year risk of dropping off the waitlist due to tumor progression or death [33]. The impact of pre-transplant therapy in this study is unknown but it does highlight the significant risk of waitlist drop-out and regional variation in transplant rates for HCC. In an effort to slow or halt disease progression while on the waitlist, many centers began to utilize ablative therapy such as trans-arterial chemoembolization (TACE), radiofrequency ablation (RFA) or percutaneous ethanol injection (PEI). Utilizing pre-transplant chemoembolization, Maddala et al. at the Mayo Clinic demonstrated a 15 % 6-month and 25 % 1-year waitlist drop-out [34]. Mazzaferro et al. treated 60 tumors in 50 patients with pre-transplant RFA [35]. After a median waiting time of 9.5 months there were no patients dropped due to tumor progression and the 1- and 3-year overall survival was 95 and 83 % respectively.
Although pre-transplant locoregional therapy such as TACE has a well-defined role as a “bridge” to transplant as level 1 evidence demonstrates it to have a survival advantage over supportive care alone in patients with unresectable, non-transplantable HCC [36–38], its role as neo-adjuvant therapy to provide oncologic benefit after transplantation is less clear. In a retrospective analysis from two centers, Yao and colleagues demonstrated a beneficial effect of locoregional therapy in post-transplant recurrence-free survival for patients with T2 and T3 HCC [39]. In these patients the 5-year recurrence-free survival was 93.8 % for patients who received locoregional therapy versus 80.6 % for those did not undergo pre-transplant treatment (p = 0.049). The treatment benefit was most notable in those patients with T3 tumors (Figs. 2 and 3) suggesting that preoperative therapy may add a survival benefit to those who undergo transplantation and may allow for selection of patients whose tumors have “good biology.” Another report by Bharat retrospectively evaluated 100 patients with HCC who underwent transplantation with 46 receiving pre-transplant locoregional therapy [40]. Those who underwent pre-transplant therapy had better 5-year survival (82.4 % versus 51.8 %; p = 0.01). When stratified by tumor stage, the treatment benefit was seen only in those with T2–T4 tumors. Patients with T1 tumors experienced excellent outcomes with or without neoadjuvant locoregional therapy (Fig. 4). Interestingly, 16 patients were found to have 100 % tumor necrosis on pathologic evaluation of the explanted liver. Eleven of these 16 had T2 or T3 disease and experienced better survival than those with T1 tumors (determined by pre-transplant imaging) who did not have a complete pathologic response.
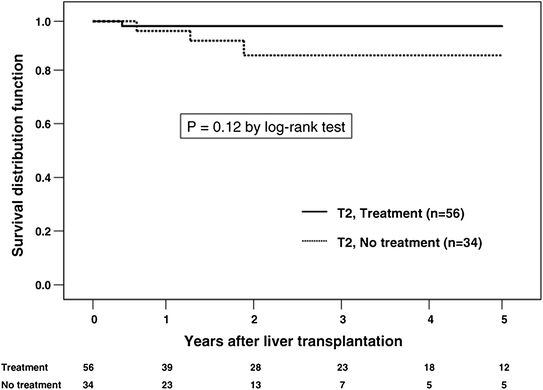
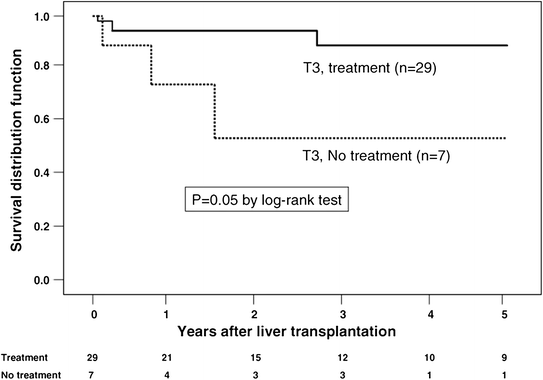
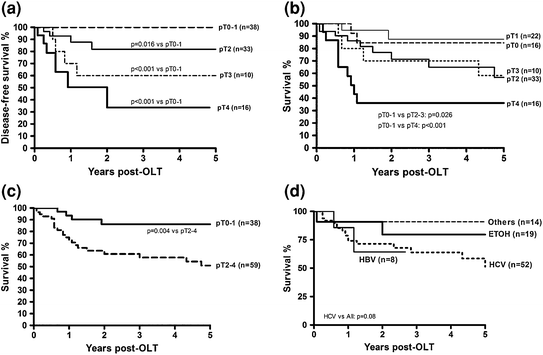

Fig. 2
The Kaplan-Meier recurrence-free survival following liver transplantation for up to 5 years according to pre-operative loco-regional treatment in the subgroup with pathologic T2 tumor stage. The difference in survival was compared by the log-rank test. The number of patients at risk at each time point is shown below the horizontal axis. (From Yao F, Kinkhabwala M, LaBerge J, Bass N, Brown Jr. R, Kerlan R, Venook A, Ascher N, Emond J, Roberts J. The Impact of Pre-Operative Loco-Regional Therapy on Outcome After Liver Transplantation for hepatocellular Carcinoma. American Journal of Transplantation. 2005 Apr;5(4 Pt1):795−804. Copyright© 2005 Wiley-Blackwell Publishing with permission.)

Fig. 3
The Kaplan-Meier recurrence-free survival following liver transplantation for up to 5 years according the pre-operative loco-regional treatment in the subgroup with pathologic T3 stage tumor. The difference in survival was compared by the log-rank test. The number of patients at risk at each time point is shown below the horizontal axis. (From Yao F, Kinkhabwala M, LaBerge J, Bass N, Brown Jr. R, Kerlan R, Venook A, Ascher N, Emond J, Roberts J. The Impact of Pre-Operative Loco-Regional Therapy on Outcome After Liver Transplantation for hepatocellular Carcinoma. American Journal of Transplantation. 2005 Apr;5(4 Pt1):795−804. Copyright ©2005 Wiley-Blackwell Publishing with permission.)

Fig. 4
Five-year survival after pre-orthotopic liver transplantation locoregional therapy (A) regardless of pathologic (p) stage; (B) after stratifying by pathologic stage; (C) and (D) after stratifying by end-stage liver disease. ETOH, ethoanol; HBV, hepatitis B virus; LRT, preorthotopic liver transplantation locoregional therapy; OLT, orthotopic liver transplantation. (From Bharat A, Brown D, Crippin J, Gould J, Lowell J, Shenoy S, Desai N, Chapman W. Pre-Liver Transplant Locoregional Adjuvant Therapy for Hepatocellular Carcinoma as a Strategy to Improve Longterm Survival. Journal of the American College of Surgeons. 2006 203(4):411−20. Copyright © 2006 Elsevier Publishing with permission.)
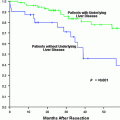
Liver resection is often considered for patients with well-compensated liver disease and HCC with transplantation reserved as “salvage” therapy for recurrence. This practice has not gained tremendous foothold in the United States due to the MELD priority given to HCC, however, it has become a commonly debated topic as the number of patients waiting on transplantation is increasing as is the prevalence of HCC. Two series have utilized this approach in transplant candidates and demonstrated differing results. The first did not reveal inferior survival results, morbidity, or early mortality for those who underwent secondary liver transplantation after resection compared to patients who underwent primary liver transplantation (Fig. 5) [41]. A second study by Adam et al. found that secondary liver transplantation after resection was associated with a much higher operative mortality, tumor recurrence, and lower 5-year post transplant survival (41 % versus 61 %; p = 0.03) (Fig. 6) [42]. Ninety-eight patients who underwent resection were transplant eligible and tumor recurred in 69 (70 %). Notably, only 17 patients (25 %) of the 69 had transplantable tumor recurrence. The 5-year overall survival of those who underwent resection of transplant eligible tumors was significantly less than that of patients who underwent primary transplantation (50 % versus 61 %; p = 0.05) (Fig. 7). A Markov model evaluating the harm and benefit of primary liver resection with salvage transplantation for HCC found that primary transplantation offered a greater life-expectancy as long as 5-year post-transplant survival rates remained greater than 60 % [43]. Based on an estimated 10 % proportion of patients on the waiting list with HCC and a median time to transplant of 3 months, the harm caused to resected patients was higher than the benefit of having those livers reallocated to the wait-listed population.
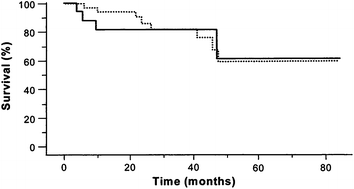
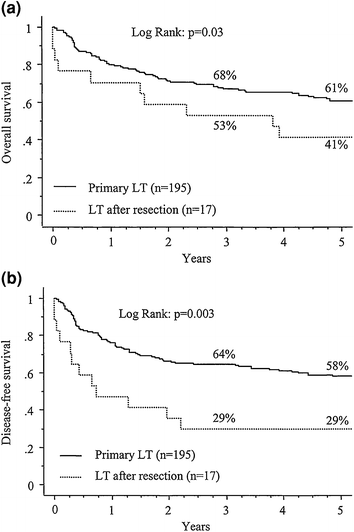
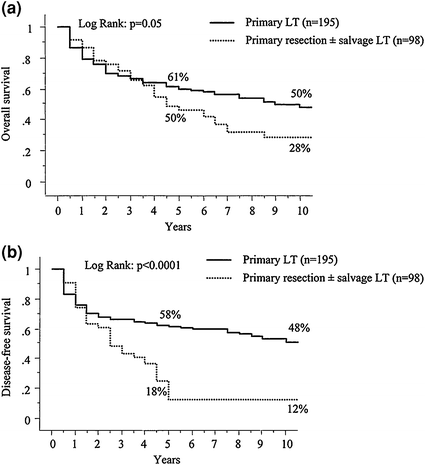

Fig. 5
Kaplan-Meier survival plots for PLT (Primary Liver Transplant) versus SLT (Secondary Liver Transplant) groups. The survival rates measured from the time of liver transplantation in the group of patients who underwent primary (solid line) versus secondary (dashed line) liver transplantation for HCC. There was a single death (5.6%) in the first 30 days postoperatively in the SLT group and 4 postoperative deaths (5.7%) in the PLT group. Patients who died in the postoperative period were excluded. (From Belghiti J, Cortes A, Abdalla E, Régimbeau J, Prakash K, Durand F, Sommacale D, Dondero F, Lesurtel M, Sauvanet A, Farges O, Kianmanesh R. Resection Prior to Liver Transplantation for Hepatocellular Carcinoma. Annals of Surgery. 2003; 238(6):885–92. Copyright © 2003 Lippincott Williams & Wilkins with permission.)

Fig. 6
Comparison of survival between primary and secondary transplantation for HCC on cirrhosis. (A) overall survival; (B) disease-free survival. (From Adam R, Azoulay D, Castaing D, Eshkenazy R, Pascal G, Hashizume K, Samuel D, Bismuth H. Liver Resection as a Bridge to Transplantation for Hepatocellular Carcinoma on Cirrhosis: A Reasonable Strategy? Annals of Surgery. 2003; 238(4):508–18. Copyright © 2003 Lippincott Williams & Wilkins with permission.)

Fig. 7
Comparison of survival between primary resection with possible transplantation and primary transplantation for HCC on Cirrhosis (A) overall survival and (B) disease-free survival. (From Adam R, Azoulay D, Castaing D, Eshkenazy R, Pascal G, Hashizume K, Samuel D, Bismuth H. Liver Resection as a Bridge to Transplantation for Hepatocellular Carcinoma on Cirrhosis: A Reasonable Strategy? Annals of Surgery. 2003; 238(4):508-18. Copyright © 2003 Lippincott Williams & Wilkins with permission.)
6 Expanding the Milan Criteria
Many patients are denied the opportunity for liver transplantation because Milan Criteria restricts the use of transplantation to those patients with very early disease. Several groups have challenged these restrictions either through expanding the size criteria imposed by Milan or by using liver directed therapy in an attempt to “down-stage” patients with advanced tumors to within criteria [30, 44–47]. Most notable has been the group from the University of California San Francisco (UCSF) who have proposed criteria of a single tumor ≤6.5 cm or up to 3 tumors the largest ≤4.5 cm and total tumor diameter ≤8 cm without gross vascular invasion [47]. These numbers were derived from explant tumor characteristics as the authors noted that explant pathology often revealed understaging by preoperative cross-sectional imaging; however, this did not necessarily result in inferior outcome. Of the 168 patients in the initial report, the 5-year recurrence-free survival was 90 % for the 130 patients with a preoperative tumor stage within Milan versus 94 % for the 30 patients who met the UCSF criteria but exceeded Milan (p = 0.58). These criteria were further evaluated in a series of 467 patients who underwent transplantation at UCLA [30]. Based on pre-transplant imaging, 173 patients were within Milan criteria, 185 were beyond Milan but within UCSF criteria, and 109 were outside of UCSF criteria. The 5-year patient survival was 79 % for those meeting Milan versus 64 % for those beyond Milan but within UCSF (p = 0.061). Based on explant pathology, the survival of those meeting Milan Criteria versus those meeting only UCSF was 86 % versus 81 % at 5 years, respectively.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree