Fig. 10.1
Type I and type II FLT3 inhibitors. The binding of FL to the extracellular domain of wild-type FLT3 leads to its dimerization, stabilizing a conformation in an active conformation. FLT3-ITD takes a form of inactive conformation, while KDMs destabilize the inactive conformation of FLT3 in favor of the active conformation. Type I inhibitor can bond to both active and inactive conformations, while type II inhibitor cannot bind to active conformation
10.3 First-Generation FLT3 Inhibitors
After the discovery of FLT3 mutations, tyrosine kinase inhibitors (TKIs), which have a potency to inhibit the FLT3 kinase, were firstly subjected to clinical trials (Fig. 10.2) [33].
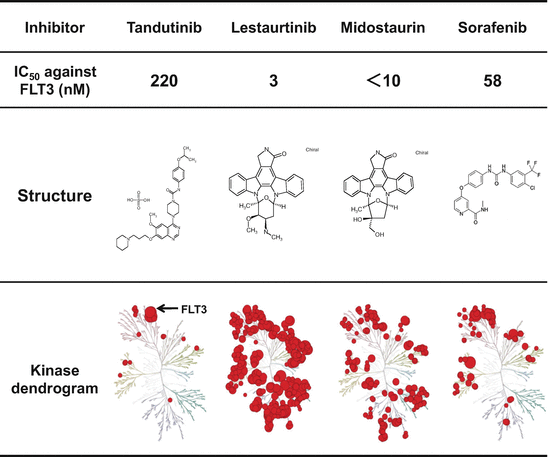

Fig. 10.2
Structure and inhibitory activity of first-generation FLT3 inhibitors. Tandutinib and sorafenib are type II inhibitor. Lestaurtinib and midostaurin are type I inhibitor, but have lower selectivity against FLT3
Tandutinib (Millennium) is a derivative of quinazoline. Although this compound has a high selectivity against FLT3, the IC50 value (220 nM) is lower than the other FLT3 inhibitors [34]. Furthermore, it inhibits the FLT3-ITD, but not FLT3-KDM, indicating a type II inhibitor [35]. In a Phase 1 study of 40 patients with relapsed or refractory AML, three patients had 40–50% reduction in the number of bone marrow (BM) blasts. In this study, no adverse effects were observed, while the peak plasma concentration of this compound did not reach to the biologically effective level. In a Phase 2 study of 25 patients with relapsed or refractory AML harboring FLT3-ITD, a decrease of peripheral and BM leukemia cells was observed in seven of the 15 patients, while clinical response could not be evaluated in eight patients because of rapid disease progression or the toxicity, such as ptosis and QTc prolongation [36]. Therefore, further clinical development was discontinued.
Sunitinib (Pfizer) is a derivative of indolinone and has been approved for renal cell carcinoma, gastrointestinal stromal tumor (GIST), and neuroendocrine tumor (NET) in Japan. It belongs to a type I inhibitor, has a unique inhibiting property to tyrosine kinases, and inhibits KIT, PDGFR, and KDR kinases more sensitively than FLT3 kinase (Fig. 10.2). In a Phase 1 study of 15 patients with advanced AML, the tentative reduction of peripheral blast cells was observed in seven patients [37]. Since two patients died of cardiotoxicity and the plasma concentration was brought to the biologically effective level, a further clinical development for AML has been discontinued.
Lestaurtinib (Cephalon) was derived from indolocarbazole. This compound is a type I inhibitor with a high potency against FLT3 kinases (IC50 is 3 nM), but has potencies against multiple kinases [38, 39]. In a Phase 2a study of 12 AML patients with FLT3 mutations, the reduction of peripheral blast cells under 5% or the loss of BM blasts was observed in four patients, while the complete remission (CR) was not achieved in any patients [40]. Since this compound is also a derivative of indolocarbazole, the plasma concentration is lower than expected. The clinical efficacy of this compound alone seemed to be limited, so a Phase 2 study with a combination of lestaurtinib and conventional chemotherapeutic agents was conducted [41]. In this study, 224 patients with the first relapsed AML harboring FLT3 mutations were randomly assigned to chemotherapy (MEC or high-dose AraC) alone or a combination of chemotherapy and lestaurtinib. The CR/CRp rate in the combination group (16%) was not statistically better than that in the chemotherapy group (21%). Based on these results, further clinical development of lestaurtinib has been discontinued.
Sorafenib (Bayer) is a type II multikinase inhibitor, which has potency against RAF-1, VEGFR, PDGFR, KIT, and FLT3, and has been approved for hepatocellular carcinoma and renal cell cancer (Fig. 10.2) [42]. The IC50 value against FLT3 is 58 nM. Although clinical efficacy of sorafenib monotherapy was limited to the transient blast reduction in two Phase 1 studies, a combination with chemotherapy revealed a high CR rate in the relapsed and/or refractory AML patients with FLT3-ITD mutations [43]. However, since a combination of chemotherapy and sorafenib did not show better overall and event-free survivals than a chemotherapy alone in elderly AML patients, further studies are required to evaluate the efficacy and safety of the combination therapy [44].
Midostaurin (Novartis) is a benzoylstaurosporine, initially developed as a KDR inhibitor, and belongs to a type I inhibitor (Fig. 10.2) [45]. In a Phase 2 study of 61 patients including 11 FLT3-ITD and 4 FLT3-KDM patients, half of the enrolled patients showed over a 50% reduction in the number of bone marrow blasts [46]. Although one AML patient with FLT3-ITD achieved complete remission, the duration was very short. Pharmacokinetic properties showed that the plasma concentration of this compound could not be maintained at the biologically effective level, because it consists of an indolocarbazole molecule, which is known to be highly associated with acid-α-glycoprotein (AGP) in human plasma. Notably, two patients died of pulmonary edema, which was considered a drug-induced toxicity. Since it was concluded that a monotherapy of midostaurin did not have sufficient clinical activity in AML patients with FLT3 mutations, a combination with chemotherapeutic regimens is evaluated. A randomized Phase 3 study (CALGB 10603/RATIFY study) was conducted for evaluating the superiority of midostaurin in addition to the conventional induction and consolidation therapies, and the interesting results were reported at the 57th Annual Meeting of ASH in 2015 . Between May 2008 and October 2011, 717 patients (555 FLT3-ITD; 162 FLT3-KDM) were randomized to either the conventional chemotherapy plus midostaurin (n = 360) or the conventional chemotherapy plus placebo (n = 357). Overall and event-free survivals were significantly superior in the midostaurin group than placebo group (P = 0.007 and 0.004, respectively) (Table 10.1). This study firstly demonstrated that the combination of chemotherapy and FLT3 inhibitor improves the long-term prognosis of the AML patients with FLT3 mutations. However, the CR rate of chemotherapy and midostaurin group (59%) was the same as that of chemotherapy and placebo group (54%), indicating that further study is required for fully understanding the significance of midostaurin in the induction therapy.
Table 10.1
Results of CALGB 10603/RATIFY study
Arm | Median month (95% CI) | P value | |
|---|---|---|---|
Overall survival | Midostaurin | 74.7 (31.5, not attained) | 0.007 |
Placebo | 26.0 (18.5, 46.5) | ||
Overall survival (SCT censored) | Midostaurin | Not attained | 0.047 |
Placebo | Not attained | ||
Event-free survival | Midostaurin | 8.0 (5.3, 10.6) | 0.0044 |
Placebo | 3.0 (1.9, 5.8) | ||
Event-free survival (SCT censored) | Midostaurin | 8.2 (5.5, 10.7) | 0.025 |
Placebo | 3.0 (1.9, 5.8) |
10.4 Second-Generation FLT3 Inhibitors
Clinical efficacies of first-generation FLT3 inhibitor monotherapy were lower than expected. In addition, several problems, such as maintaining the effective plasma concentration and serious adverse events, have been apparent to be resolved before clinical use. Since the first-generation FLT3 inhibitors were not originally screened for the sensitivity and selectivity against the activated FLT3 kinase, the second-generation FLT3 inhibitors have been developed based on the growth inhibitory effects against leukemia cells with FLT3 mutations (Fig. 10.3).
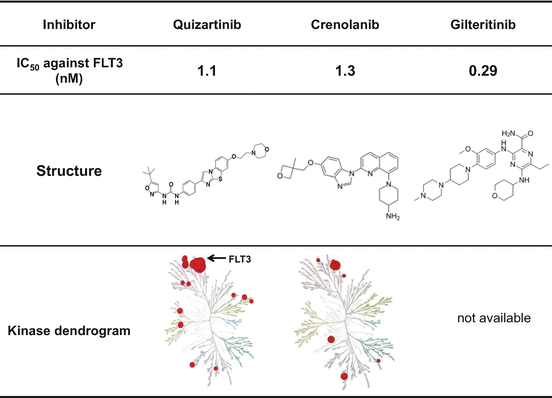

Fig. 10.3
Structure and inhibitory activity of second-generation FLT3 inhibitors. Quizartinib is a highly FLT3 selective type II inhibitor. Crenolanib and gilteritinib are type I inhibitors, and their inhibitory profiles are more FLT3 selective than lestaurtinib and midostaurin
Quizartinib (Daiichi-Sankyo) has been screened for the affinity against FLT3 using the KinomeScan technique and has a high selectivity and sensitivity to FLT3. Although the IC50 value against dephosphorylation of FLT3-ITD is 1.1 nM, this compound does not have a potency against FLT3-KDM (type II inhibitor) [33]. The preclinical study showed the high bioavailability and AUC [47]. In a Phase 1 study, the QTc prolongation was the dose-limiting toxicity, while one CR and four incomplete CR (CRi) were observed in 18 AML patients harboring FLT3-ITD. In a Phase 2 study, 40 of the 79 (50.6%) patients with relapsed and/or refractory AML harboring FLT3-ITD achieved CRp or CRi. However, no CR was observed due to the BM suppression. The BM suppression is thought to be caused by the KIT inhibition of quizartinib. In addition, the QTc prolongation was also observed even at the recommended dose, which was determined by the Phase 1 study. These results indicated the strong potency of quizartinib against AML cells harboring FLT3-ITD, while it is necessary to determine the optimal dose and/or schedule in the clinical use.
Crenolanib (Arog Pharmaceuticals) is a benzamidine quinolone derivative and developed as a potent inhibitor of PDGFR, while subsequent analysis revealed that it has a potency against both FLT3-ITD and FLT3-KDM (Fig. 10.3) [48]. Crenolanib is a type I inhibitor and has the same potency against FLT3-ITD (IC50 is 1.3 nM) as quizartinib. Although it has a potency against FLT3-KDM, inhibitory activities are different in the types of FLT3-KDM. Of note is that crenolanib inhibits all types of D835-mutated FLT3 kinases, but does not F691L-mutated FLT3 (Table 10.2). Furthermore, recent study demonstrated that the combination with type II inhibitor sorafenib increases antileukemia activity, suggesting that simultaneous type I and type II inhibition may increase efficacy of leukemia cells regardless mutation types of FLT3 [49]. Clinical efficacy and safety of crenolanib is now evaluated by monotherapy and combination therapy with conventional chemotherapy or sorafenib.
Table 10.2
Inhibitory activity of crenolanib against mutant FLT3 kinases
Mutation type | IC50 (nM) |
|---|---|
ITD | 1.3 |
D835Y | 6.9 |
D835F | 6.5 |
D835H | 19.8 |
D835N | 4.3 |
D835V | 2.3 |
ITD/D835Y | 8.7 |
ITD/F691L | 67.8 |
Gilteritinib (ASP2215, Astellas) has been developed for a selective inhibitor of AXL and FLT3. It was reported that gilteritinib has a potency against both FLT3-ITD and D835-mutated FLT3 (type I inhibitor). The preliminary data of Phase 1/2 study was reported at the Annual Meeting of ASCO in 2015. Although gilteritinib was well tolerated from 20 to 300 mg, DLT (Grade 3 diarrhea and AST/ALT elevation) was observed in two patients at 450 mg. The MTD was, therefore, determined as 300 mg. It was also reported that composite complete remission (CRc; CR, CRp, and CRi) and overall response (CRc plus partial remission) were observed in 47.2% and 57.5%, respectively, in 106 relapsed or refractory AML patients with FLT3 mutations who were treated with more than 80 mg of gilteritinib. Randomized Phase III study of gilteritinib versus salvage chemotherapy in patients with relapsed or refractory AML with FLT3 mutation is now conducted.
10.5 Resistance of FLT3 Inhibitors
To date, several FLT3 inhibitors were subjected to the clinical trials. Although each FLT3 inhibitor has a characteristic property about sensitivity and selectivity, resistance has been apparent regardless the character through the early clinical studies. Resistant mechanisms of FLT3 inhibitors are classified into primary and secondary resistances (Table 10.3) [50, 51]. The primary resistance includes a different potency against types of FLT3 mutations, other activating signals, and the lower potency against leukemia stem cells. Although FLT3 inhibitors were classified into type I and type II inhibitor based on the potencies against FLT3-ITD and FLT3-KDM, it remains unclear which type of inhibitor improves the prognosis of the patients with FLT3 mutations. Since the first-generation type I inhibitors, such as lestaurtinib and midostaurin, have potency against many kinases in addition to FLT3, adverse events by off-target effects were thought to be a disadvantage of type I inhibitor. Therefore, clinical efficacy of more FLT3 selective type I inhibitors, such as crenolanib and gilteritinib, is highly expected. However, recent results of Phase 3 study demonstrated that an addition of midostaurin to chemotherapy significantly improved both overall and event-free survivals in AML patients with FLT3 mutations; further study is required to clarify how type I inhibitors should be used for getting the best clinical result.
Table 10.3
Primary and secondary resistant mechanism of FLT3 inhibitor
Primary resistance | Secondary resistance |
|---|---|
Low potency against FLT3-KDM | Acquiring resistant mutations |
Other activating signals | FL stimulation |
Low potency against leukemia stem cells | Overexpression of FLT3 |
Table 10.4
Comparison of GI50 values of FLT3 inhibitors in the FL present condition
FLT3 inhibitors
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|
|---|