Hodgkin’s disease (HD) is a fatal disorder with the unique histologic features of few dysplastic Hodgkin- and Reed-Sternberg (HRS) cells surrounded by an abundance of nonatypical bystander cells in primary biopsies. By using the first Hodgkin cell line L428 the cytokine receptor CD30 was discovered. CD30 proved to be an excellent target for the diagnoses of CD30+ malignancies and for monoclonal antibody therapy in patients with these malignancies because of its highly restricted expression in healthy individuals. Recently, a new anti-CD30-toxin-drug-conjugate consisting of an anti-CD30 monoclonal antibody bound to the nonimmunogenic toxin auristatin E with a newly designed linker was generated.
Key points
- •
Hodgkin’s disease (HD) is a fatal disorder with the unique histologic features of few dysplastic Hodgkin- and Reed-Sternberg (HRS) cells surrounded by an abundance of nonatypical bystander cells in primary biopsies.
- •
By using the first Hodgkin cell line L428 the cytokine receptor CD30 was discovered.
- •
CD30 proved to be an excellent target for the diagnoses of CD30+ malignancies and for monoclonal antibody therapy in patients with these malignancies because of its highly restricted expression in healthy individuals.
- •
Recently, a new anti-CD30-toxin-drug-conjugate consisting of an anti-CD30 monoclonal antibody bound to the nonimmunogenic toxin auristatin E with a newly designed linker was generated.
Why was it essential to establish a Hodgkin cell line?
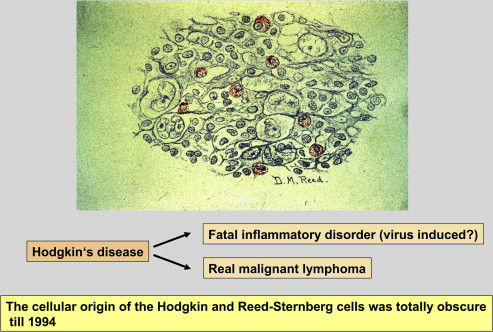
When the histology of Hodgkin disease (HD) was investigated (1898 first by Sternberg and 4 years later by Reed ), an unusual cellular composition for a fatal disorder was observed ( Fig. 1 ). Most of the cells in the affected lymph nodes proved to be nonatypical bystander cells (ie, lymphocytes, plasma cells, histiocytes, neutrophilic and/or eosinophilic granulocytes, and others). The atypical large mononuclear and multinuclear blastoid cells, called Hodgkin and Reed-Sternberg (HRS) cells, were found in the minority, ranging from 0.1% to 1% of all cells present in the specimen. Because of this, it was thought that HD is more likely an infectious disease or an inflammatory process than a true neoplasm. The rarity of the HRS cells and the abundance of surrounding bystander cells made the investigation of HRS cells with special techniques impossible.
Why was it essential to establish a Hodgkin cell line?
When the histology of Hodgkin disease (HD) was investigated (1898 first by Sternberg and 4 years later by Reed ), an unusual cellular composition for a fatal disorder was observed ( Fig. 1 ). Most of the cells in the affected lymph nodes proved to be nonatypical bystander cells (ie, lymphocytes, plasma cells, histiocytes, neutrophilic and/or eosinophilic granulocytes, and others). The atypical large mononuclear and multinuclear blastoid cells, called Hodgkin and Reed-Sternberg (HRS) cells, were found in the minority, ranging from 0.1% to 1% of all cells present in the specimen. Because of this, it was thought that HD is more likely an infectious disease or an inflammatory process than a true neoplasm. The rarity of the HRS cells and the abundance of surrounding bystander cells made the investigation of HRS cells with special techniques impossible.
The establishment of the L428 cell line
To obtain pure HRS cells in large quantities, many laboratories tried to establish long-term in vitro cultures of primary HRS cells when in the 1960s and 1970s suitable culture conditions like RPMI media together with fetal calf serum became available. All attempts by Zech and colleagues, Kaplan and Gartner, and many others were unsuccessful. The same was true for the 427 culture attempts by Volker Diehl and his group between 1969 and 1978 at the Radiumhemmet/Karolinska Sjukhuset in Stockholm/Sweden and later in Hannover/Germany. Most of the culture attempts resulted in Epstein-Barr virus-transformed lymphoblastoid cell cultures. After his relocation to Hannover, Diehl continued with the attempts to establish in vitro cultures of Hodgkin-Reed-Sternberg cells and succeeded with his team in 1979 in growing for the first time a permanently growing Epstein-Barr virus-negative cell line from a pleural effusion of a young female patient with nodular sclerosing HD ( Table 1 ).
| Year | Histology | Stage | Therapy | Result |
|---|---|---|---|---|
| 2/1972 | Nod. sclerosis | II, B supracl. LN, mediast. LN | Ext. RT | CR |
| 2/1974 | Relapse, NS | IVE, B, chest wall infiltration, pleural effusion | Chemo-radiotherapy: COPP-ABVD+ RT | PR→ Progressive disease |
| 11/1974 | Progression | → | Palliation Pleural tappings 2–4 wk before death a | Death |
a Time points of pleural tappings for in vitro cell cultures.
Features of the L428 Hodgkin Cell Line
This cell line was designated L428, because it was the 428th culture attempt. The in vitro proliferating cells proved to be aneuploid, carrying several marker chromosomes (1p+, 2p+, 6q+, 7q+, 9p+, 11q−, 13p+, 21q−). The total number of chromosomes per cell amounted to 48–50. These findings demonstrated that the L428 cell-line cells were monoclonal and derived from a very atypical cell population. Immunophenotypical studies revealed an absence of B-cell-, T-cell-, and macrophage markers ( Fig. 2 A ). Considering these findings, it was tempting to assume that the L428 cells were direct derivatives from HRS cells. In addition, the disease report of the donor patient (see Table 1 ) of the L428 cell line supported this conclusion.
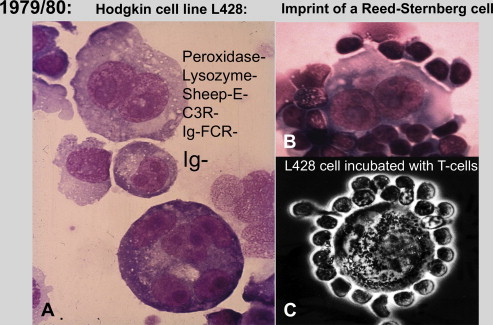
Despite the mentioned findings and especially because of the disappointing publications by Kaplan and Gartner and Long and colleagues, there was general hesitation in the scientific community to accept the L428 cell line as a true Hodgkin cell line. In this situation Diehl approached Stein with the request to establish arguments that could help to show that the L428 cell line cells were real derivatives from in vivo HRS cells.
What Stein did first was to incubate the L428 cells with his own T cells. This experiment showed that the L428 cells bind T cells in rosette formation like primary HRS cells (Stein H, unpublished observation, 1980) (see Fig. 2 C) and was an important further criterion for the assumption that the L428 cells represent true HRS cells.
Discovery of the CD30 antigen and its application in research, diagnostic, and therapy
Detection of HRS-cell-related Antigens by Rabbit Polyclonal Antisera
To detect HRS-cell-specific antigens, Stein’s team immunized rabbits with the L428 cell-line cells. Two rabbit antisera selectively immunostained HRS cells following absorption with neutrophils and Daudi-cell-line cells. One antiserum stained the nuclei and the other antiserum stained the cytoplasm of HRS cells ( Fig. 3 ).
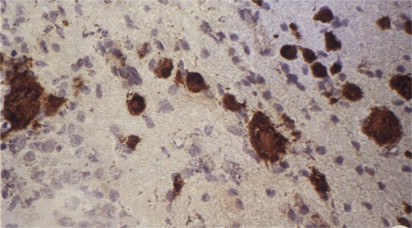
Detection of the Proliferation-Associated Antigen Ki-67 and the HRS-cell-Related Antigen Ki-1
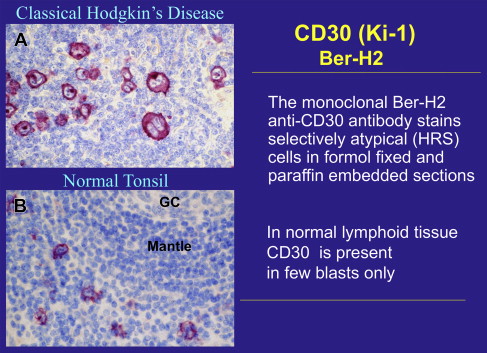
Ki-1 by the monoclonal antibody approach
To generate a permanent source of antibodies that react selectively with HRS cells, the L428 cells were subjected to the monoclonal antibody (moab) approach. More than 3000 hybridoma supernatants were screened by immunostaining frozen sections of a Hodgkin case, a tonsil, and L428 cells. Two monoclonal antibodies reactive with HRS cells could be identified. One moab called Ki-67 did not only react with the nuclei of HRS cells but also reacted with the nuclei of germinal center cells. Further studies confirmed that Ki-67 is a proliferation-associated antigen that is expressed throughout the whole cell cycle but not in resting cells. The other moab, called Ki-1, confirmed the existence of an antigen that is highly restricted to HRS cells and is present on the surface membrane and the cytoplasm of HRS cells ( Fig. 4 A ). In normal lymphoid tissues the Ki-1 antigen was encountered only on a few mononuclear blastoid cells usually located in the perifollicular area of secondary follicles ( Fig. 4 B).