Fibroblast Growth Factor Receptor (FGFR) Inhibitors
Catherine Pisano
Christopher Iriarte
Nicole R. LeBoeuf
Fibroblast growth factors (FGFs) and their receptors (FGFRs) play an important role in normal cell biology, including early embryogenesis, organ formation, wound healing, and angiogenesis. They also play a vital role in cell proliferation, mitogenesis, cell migration, and cell death.1 Mutations in or dysregulation of FGF/FGFR have been observed in a wide range of solid organ tumors including cholangiocarcinoma, urothelial carcinoma, non-small cell lung cancer, and glioblastomas.2,3,4,5,6
To date, there are five known FGFRs (FGFR1, FGFR2, FGFR3, FGFR4, and FGFR5) which have more recently become a target of anticancer therapy.2 Currently, erdafitinib (FGR2 and FGR3 inhibitor) has FDA approval for the treatment of metastatic urothelial carcinoma, and pemigatinib (FGFR2 inhibitor) and infigratinib (FGFR2 and FGFR3 inhibitor) have FDA approval for the treatment of cholangiocarcinoma. Other FGFR inhibitors pending FDA approval include derazantinib (FGFR 1-3 inhibitor) and futibatinib (FGFR1-4 inhibitor).7 FGFRs have been associated with distinct cutaneous adverse reactions including xerosis and xerostomia, nail changes, alopecia, stomatitis, hand-foot skin reaction (HFSR), calcinosis cutis, and calciphylaxis.2
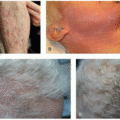
18% of patients receiving FGFR inhibitors experience xerosis.8 Xerostomia is seen in up to 59% of cholangiocarcinoma patients and up to 46% of urothelial cancer patients treated with FGFR inhibitors.9,10
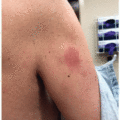
FGFR inhibitors also cause painful stomatitis; up to 65% of patients receiving erdafitinib are reported to develop stomatitis, and 18% of those are >grade 3 reactions9 (Figure 36.1).
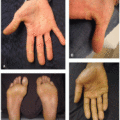
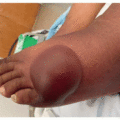
Nail changes including onycholysis, paronychia, onychomadesis, and onychoschizia are another common adverse event seen with FGFR inhibitor use (Figures 36.1 and 36.2). Nail adverse events usually develop within the first 1 to 2 months following initiation of FGFR inhibitor treatment.2 Onycholysis has been reported in up to 18% of patients receiving FGFR inhibitor therapy.11 Paronychia and onychomadesis were observed in 7% and 18% of patients, respectively.12 Clinically, there sometimes appears to be hyperkeratosis and/or inflammation of the nail bed, leading to visualized changes.
Alopecia can range from grade 1 alopecia, which encompasses thinning or patchy hair loss, to grade 2 alopecia, which is defined as loss of more than 50% of hair. Up to 46% of cholangiocarcinoma patients and up to 39% of urothelial carcinoma patients on FGFR inhibitors experience grade 1 or 2 alopecia.13,14 Additionally, there have been reports of eyelash trichomegaly with FGFR inhibitor use.15
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree