Granulocyte count <500/μl or <1,000/μl with predicted decline to ≤500/μl
Single oral temperature of >38.3 °C
or ≥38.0 °C × 2 within 12 h
or ≥38.0 °C over ≥1 h
No obvious noninfectious origin
Adverse reaction to blood products, cytokines, other drugs
Patients with a higher risk of a more complicated clinical course of infection should initially be treated with intravenous antimicrobial therapy. Current IDSA guidelines recommend monotherapy with an antipseudomonal beta-lactam such as cefepime, piperacillin-tazobactam, or a carbapenem (meropenem, imipenem-cilastatin) in complicated situations (sepsis, pneumonia) or a duotherapy with an antipseudomonal beta-lactam antibiotic plus an aminoglycoside as appropriate choices for initial empirical therapy in this setting [9]. In general, the combination of the beta-lactam with an aminoglycoside is not required [5, 22]. Fluoroquinolones such as ciprofloxacin are not recommended for initial empirical intravenous monotherapy with respect to inferior efficacy [20] and the considerable emergence of resistance among gram-negative bacilli against quinolones in the past decade [1].
It is not recommended to include an antibacterial agent with specific activity against gram-positive cocci such as vancomycin in the empiric first-line regimen in patients with fever of unknown origin. This combination may be considered in patients with clinical signs of a gram-positive infection (see below) or those with hemodynamic instability in a setting with high prevalence of methicillin-resistant Staphylococcus aureus (MRSA).
The initial empiric addition of a broad-spectrum antifungal agent such as voriconazole is not justified in FUO patients, because it does not increase response rates and does not prevent fungal breakthrough infections [19].
9.6 Modification of Antimicrobial Treatment in Patients with Persisting Fever After 4 Days of Initial Therapy
A daily clinical reassessment of neutropenic patients with fever of unknown origin receiving empiric antibacterial therapy is essential. Clinical or radiological findings indicating a specific spectrum of microorganisms have been summarized in Table 9.2.
Table 9.2
Typical clinical findings at specific physical sites and predominant microbial pathogens involved
Clinical symptoms | Typical pathogens |
|---|---|
Erythema/pain at venous access | Coagulase-negative staphylococci |
Mucosal ulcers | Alpha-hemolytic streptococci, Candida spp. |
Single point-like erythemas | Gram-positive cocci, Candida spp. |
Necrotizing skin lesions | Pseudomonas aeruginosa, Aspergillus spp. |
Retinal infiltrates | Candida spp. |
Diarrhea, meteorism | Clostridium difficile, norovirus |
Enterocolitis, perianal lesion | Polymicrobial incl. anaerobes |
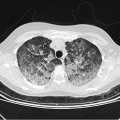
Lung infiltrates ± sinusitis | Aspergillus spp., zygomycetes, P. jirovecii |
In general, a decision on maintaining or modifying the initial antimicrobial regimen is made after 96 h, after all cultures have tested negative and no clinical or imaging finding enables a more targeted anti-infective treatment. At this point, three options have to be considered: (1) leaving the antimicrobial treatment regimen unchanged, (2) switching to another antibacterial agent, or (3) adding a broad-spectrum antifungal.
Before this decision is made, all potential sites of infection (see Table 9.2) must be reexamined, blood cultured be taken once more (including those drawn from the venous catheter), and the patient be sent to a thoracic CT scan. In individual low-risk patients who are clinically stable and expect the recovery of their neutrophil counts, this procedure might be suspended, but for all other patients, it is strongly recommended. Approximately 50 % of patients without any clinical finding indicative of pneumonia will show pulmonary infiltrates on CT scans [10], which are frequently missed on plain chest radiographs. It can be considered to include paranasal sinuses in CT studies; however, their benefit for treatment decisions in asymptomatic, persistently febrile neutropenic patients has not been conclusively shown as yet. This is different in patients who show pulmonary infiltrates at this time, because pathological findings on CT scans of paranasal sinuses may facilitate the diagnosis of invasive fungal infection [11].
In patients with persistent fever of unknown origin, i.e., with unremarkable results of the abovementioned diagnostic procedures, who are clinically stable, and in whom laboratory parameters such as C-reactive protein and interleukin-6 have not significantly increased in comparison to baseline diagnostics, the antibacterial treatment regimen should be continued without modification [9], irrespective of their risk group allocation. In low-risk patients in whom a treatment modification is regarded necessary, because clinical or laboratory findings indicate an uncontrolled febrile complication (clinical instability, increasing proinflammatory laboratory parameters), a change of antibacterial treatment should be prompted. In patients initially treated with ciprofloxacin and amoxicillin-clavulanate, piperacillin-tazobactam may be a suitable second-line regimen. In patients treated with piperacillin-tazobactam or cefepime, a switch to imipenem or meropenem should be considered.
In high-risk patients in whom treatment modification is clinically indicated, the antimicrobial regimen should be escalated by the addition of a broad-spectrum antifungal agent, i.e., liposomal amphotericin B or caspofungin (both licensed for this indication). The modification of antibacterial therapy is debatable. It appears justified to switch from piperacillin-tazobactam or cefepime to imipenem or meropenem, because these carbapenems may be effective against pathogens non-susceptible to the former agents. In contrast, in patients already given imipenem or meropenem for first-line therapy, a modification of antibacterial therapy is more speculative. Although apparently logical, the addition of a glycopeptide (vancomycin or teicoplanin) has not been shown to be superior to the addition of placebo [4, 6]. In institutions with a high prevalence of multiresistance among gram-negative microorganisms, the addition of an aminoglycoside might be considered.
Empiric antiviral treatment or the administration of macrolide antibiotics or trimethoprim-sulfamethoxazole should be omitted.
9.7 Approach to Patients with Clinically Documented Infections
A primary (“preemptive”) modification of antimicrobial therapy may be beneficial in patients with distinct clinical symptoms, particularly in those with pulmonary infiltrates, a venous catheter-associated infection, and central nervous system or abdominal/perianal infection (Table 9.2). Those scenarios are addressed in separate chapters of this textbook.
9.8 Targeted Treatment in Microbiologically Documented Infections
Microbiological findings can be extremely helpful in order to target antimicrobial treatment; however, they should always be critically interpreted with respect to their causal relationship to the clinical picture of infection. Irrespective of their potentially pathogenic character, some microorganisms may represent nothing but residual flora after antibiotic therapy, e.g., enterococci isolated from oropharyngeal samples. Other findings may point at a separate relevant infection but are irrelevant for the infection that gave reason for taking the sample, e.g., coagulase-negative staphylococci repeatedly isolated from blood cultures in a patient with pneumonia. Moreover, two infections may be present concomitantly or sequentially, e.g., invasive pulmonary aspergillosis in a patient with pneumonia caused by Klebsiella spp. not responding to appropriate antibacterial treatment.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree