Fever and Sweats
Kaci Osenga
James F. Cleary
Temperature and Associated Symptoms
Temperature has long been used in clinical medicine and was included in the cardinal signs of inflammation described as “tumor, rubor, dolor, and fever.” The measurement of temperature by a thermometer, in the sublingual, subaxillary or rectal locations, is not a clinical skill practiced regularly by doctors. In most cases, temperature is recorded by other health workers or by patients themselves. Normal body temperature is considered to be 37°C, the average core temperature for an adult population.
Temperature is tightly controlled within a narrow range in each individual. Fever is defined as any elevation in the core body temperature above the normal and results from the upregulation of body temperature. More commonly, a temperature greater that 38°C is considered a clinically significant fever. In oncologic practice and many clinical studies, a significant fever is defined as a single temperature reading greater than 38.5°C or three readings (at least an hour apart) of more than 38°C. The term fever (or pyrexia) of unknown origin, FUO (PUO), is used commonly and often incorrectly in the daily practice of medicine. An FUO is defined as an illness lasting at least 3 weeks with a fever higher than 38°C on more than one occasion and which lacks a definitive diagnosis after 1 week of evaluation in a hospital (1).
Fever is often accompanied by other symptoms, including sweating and rigors. Sweating, when it accompanies fever, is a cooling response by the body wherein heat is released from the body as it evaporates water on the skin’s surface. Rigors and shivering also contribute to temperature control and are rapid muscle spasms designed to increase heat production within the body. For adult humans and most large mammals, shivering is the major means of increased heat production in response to a cold environment. Nonshivering thermogenesis, a process involving heat production in brown adipose tissue, is important in the temperature control of infants.
Control of Temperature
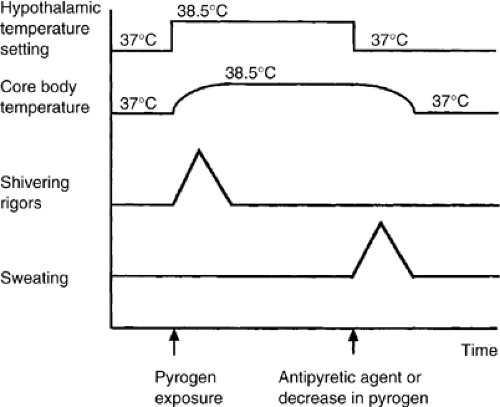
It is proposed that core body temperature is controlled by neurologic mechanisms centered at the anterior hypothalamus. The onset of fever in patients results from an elevation of the body’s regulated set-point temperature through a resetting of the temperature “gauge” in the hypothalamus (2). This may be caused by various drugs or by endogenous pyrogens. As a result of the reset hypothalamic temperature, the body increases the core body temperature to this new level (Fig. 9.1) through shivering or nonshivering thermogenesis. The continued presence of pyrogen at the hypothalamus results in the maintenance of this higher temperature. Eventually, either as a result of a decrease in the quantity of pyrogen or the administration of an antipyretic, the hypothalamic temperature is reset to a lower or normal level. The core body temperature is therefore lowered through sweating. This control mechanism may be suppressed in patients administered steroids or anti-inflammatory agents. Older patients may not be able to mount the anticipated febrile response.
The endogenous pyrogens that are responsible for the onset of fever are largely derived from monocytes and macrophages. These cells, as a result of challenge by either endotoxin or infective sources, release tumor necrosis factor (TNF) and interleukin (IL)-1 β. Their production is part of the complex cascade that results in the stimulation of other cytokines, such as iIL-6, IL-8, and changes in prostaglandin metabolism. Serum levels of IL-6 and IL-8 have been found to correlate with core body temperature in patients with febrile neutropenia (3). The ultimate end point of this cascade is the activation of granulocytes, monocytes, and endothelial cells. Although fever appears to be associated with enhanced function of the immune system, it must nonetheless be noted that a direct connection
between such phenomena and a beneficial effect of fever on outcome of infections has not been established. Fever, in fact, may be deleterious in the setting of autoimmune disorders or infections (4).
between such phenomena and a beneficial effect of fever on outcome of infections has not been established. Fever, in fact, may be deleterious in the setting of autoimmune disorders or infections (4).
Etiology of Fever in Cancer Patients
Fever is commonly seen in patients with cancer, both in those with and without infection. The wide range of etiologies of fever in patients with cancer will be considered in relation to the pathophysiology of fever in these patients.
Tumor
Fever associated with tumor is believed to be associated with the release of pyrogens, either directly from a tumor or from tumor stimulation of immunologic mechanisms that cause an elevation in temperature through action on the anterior hypothalamus. The classic association of fever to particular tumor diagnoses relates more to tumors associated with a diagnosis of FUO (Table 9.1). In the combined results of six studies documenting the etiology of FUO, 23% of adults meeting the defined diagnosis were found to have malignancy as the cause (8% of children) (5). In another study of 111 elderly (age >65 years) patients with FUO, 26 had associated malignancy, with 15 patients diagnosed with lymphoma, and 4 with renal cell carcinoma (6). Almost 7000 cancer patients with fever were reviewed by Klastersky et al., and only 47 (0.7%) fit the diagnostic criteria for an FUO (7). Twenty-seven of the 47 had leukemia or lymphoma, with disease rather than infection being the cause of the fever in only 11. Tumor was responsible for fever in seven patients with widespread metastatic carcinomas; in six of these, large liver metastases were present.
Table 9.1 Tumors Classically Associated With Fever | |
|---|---|
|
Table 9.2 Incidence of Fever Without Evidence of Infection at Autopsy in Patients of Different Primary Tumor Types | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
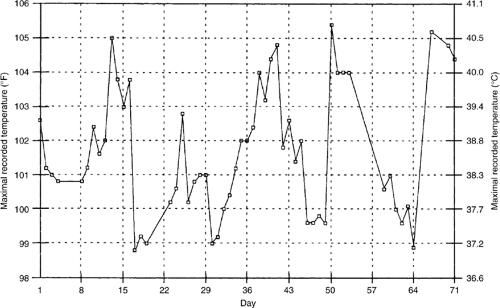
Hodgkin’s disease has classically been associated with the Pel-Ebstein fever (Fig. 9.2) where a patient experiences 3- to 10-day cycles of fever alternating with periods of normal temperature (8). Although the presence of fever is an important prognostic indicator in patients with Hodgkin’s disease, there has been some discussion (9) about the value of Pel-Ebstein fever as a diagnostic tool particularly because the original description of the Pel-Ebstein fever was made in two patients who were subsequently found, on pathologic review, not to have Hodgkin’s disease.
Although classical teaching is that fever is associated with particular tumors, fever also occurs in patients with many of the more common cancers (Table 9.2). Forty-one percent of those who underwent autopsy had evidence of infection as an explanation of their fever (10). The incidence of infection among the autopsied patients was 50% for acute leukemia, 75% in patients with lymphoma, and 80% in those with chronic lymphocytic leukemia. Infection was only found in a third of those with chronic myeloid leukemia, 17% with Hodgkin’s disease, and 15% of patients with lung cancer.
Infection (Including Neutropenia)
Although infection and fever can be a common presentation in patients with cancer, it is of particular concern in patients with neutropenia. Neutropenia, defined as a peripheral blood neutrophil count of < 500 per μL, results from either increased destruction or decreased production of white blood cells. Decreased production by the bone marrow may result from either disease involving the marrow or from myelosuppression by chemotherapy. The cause of fever is not identified in approximately 60–70% of patients with neutropenia (11). Risk factors for the development of fever in the setting of neutropenia have been identified and include a rapid decrease in the neutrophil count and a protracted neutropenia of <500 cells per μL or <10 days (12). Twenty percent of patients with 1 week of chemotherapy-induced neutropenia develop a fever and the rate of infection increases with lengthening periods of neutropenia. Other factors that may alter the risk of the patient with neutropenia include phagocyte function, the status of the patient’s immune system, and alterations in the physical defense barriers of the body (e.g., mucositis).
Fever and neutropenia in patients with cancer are associated with a high risk of medical complications with a death rate ranging from 4 to 12%. Twenty-one percent of patients with febrile neutropenia at the Dana Farber Cancer Center developed serious medical complications (13). The investigators identified four risk groups for patients with febrile neutropenia. Group 1 consisted of inpatients at the time of onset of fever; group 2, outpatients who developed significant comorbidity within 24 hours of presentation; group 3, outpatients with uncontrolled cancer but without serious concurrent comorbidity;
and group 4, outpatients without serious concurrent comorbidity and whose cancer was well controlled. The model was validated in 444 patients with febrile neutropenia, of whom 36% had a significant comorbidity, 27% had serious medical complications, and 8% died. Group 1 had the greatest risk and group 4 had little risk in relation to medical complications and risk (Table 9.3).
and group 4, outpatients without serious concurrent comorbidity and whose cancer was well controlled. The model was validated in 444 patients with febrile neutropenia, of whom 36% had a significant comorbidity, 27% had serious medical complications, and 8% died. Group 1 had the greatest risk and group 4 had little risk in relation to medical complications and risk (Table 9.3).
Table 9.3 Incidence of Multiple Medical Complications and Mortality in Patients With Febrile Neutropenia as Defined by Riska | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||
An important component of this study was the identification within 24 hours of onset of fever of those patients at low risk of medical complication. These low-risk patients were at risk of developing medical complications (5%) but these were either transient and asymptomatic or were heralded by at least 7 days of medical deterioration and therefore readily detectable by appropriate follow-up. Two additional risk factors—a latency period of <10 days from the time of chemotherapy administration to the onset of fever, and neutropenia and age >40—correlated with the occurrence of more frequent complications. Mucositis was associated with decreased risk of medical complications, suggesting that infection associated with mucositis may be responsive to antibiotics. The identification of a causative organism or positive blood cultures was not associated with increased risk.
In a multinational study, a risk-index score was developed to “stage” those patients with febrile neutropenia (14). Predictive factors included blood pressure, presence of chronic obstructive pulmonary disease or solid tumor, previous fungal infection in patients with hematologic malignancies, outpatient status, status of hydration, and age in relation to 60 years. On the validation set, a Multinational Association for Supportive Care in Cancer risk-index score ≥21 identified low-risk patients with a positive predictive value of 91%, specificity of 68%, and sensitivity of 71%. In a further population, the risk index accurately identified patients at low risk for complications. This risk index may be useful in the selection of patients for studies that test strategies to treat febrile neutropenia.
On the basis of a number of retrospective studies, similar definitions of low risk have been applied to pediatric patients with fever and neutropenia (15). Low risk included evidence of bone marrow recovery in culture-negative patients who were afebrile for at least 24 hours and who had no other reason to continue intravenous antibiotics in the hospital. The control
of any localized infection and the patient’s ability to return promptly in the event of fever or other complications were also necessary. In a prospective study of 70 patients who met the criteria and who were discharged home with neutropenia, none was readmitted with fever. Seven patients who were inadvertently discharged without evidence of marrow recovery were readmitted with recurrence of fever. Neutropenic children with positive cultures were also assessed to identify risk factors for bacteremia (16). Of the cases of bacteremia, 92.5% occurred in those where cancer was not controlled, were <1 year of age, <10 days past their last chemotherapy, and had no evidence of marrow recovery.
of any localized infection and the patient’s ability to return promptly in the event of fever or other complications were also necessary. In a prospective study of 70 patients who met the criteria and who were discharged home with neutropenia, none was readmitted with fever. Seven patients who were inadvertently discharged without evidence of marrow recovery were readmitted with recurrence of fever. Neutropenic children with positive cultures were also assessed to identify risk factors for bacteremia (16). Of the cases of bacteremia, 92.5% occurred in those where cancer was not controlled, were <1 year of age, <10 days past their last chemotherapy, and had no evidence of marrow recovery.
Organisms (Bacteriology)
A basic understanding of the classification and sensitivities of the different organisms is essential to understand the infection in patients with cancer. Over time there have been changes in the underlying organisms associated with febrile neutropenia as evidenced by progressive studies by the European Organization for Research and Treatment of Cancer (EORTC) group (17). Gram-negative organisms were the leading cause of infection in patients with febrile neutropenia, but their incidence has decreased from 71% of identified causative organisms during the 1973–1978 period to 31% during the 1989–1991 period. Infection by one of these gram-negative organisms—Pseudomonas aeruginosa—has been a driving force in the selection of antibiotics. However, the incidence of pseudomonas infection has also decreased over the last few years as reflected by an incidence of only 0.1% of febrile neutropenic cases at the National Cancer Institute (18). The incidence of both acute and chronic fungal infections has also increased, with up to 33% of patients with febrile neutropenia not responding to a week of antibiotic therapy, after having a systemic fungal (Candida or Aspergillosis) infection (19).
The incidence of gram-positive organisms has increased from 29% during the 1973–1978 period to 69% during the 1989–1991 period, requiring a review in treatment regimens used in patients with febrile neutropenia. Some of these gram-positive organisms such as coagulase-negative staphylococci or Corynebacterium jeikeium, represent indolent infections that are methicillin resistant and only susceptible to vancomycin, quinupristin-dalfopristin and linezolid. Other gram-positive bacteria such as Staphylococcus aureus, viridans streptococci and pneumococci may cause fulminant infections with serious complications and possibly death if not treated promptly. Gram-negative bacilli, especially P. aeruginosa, Escherichia coli and Klebsiella species, remain prominent causes of infection and must be treated with selected antibiotics (20).
Location
Infections can occur throughout the body and need to be sought carefully through history and examination. Collapse, consolidation, and superimposed infection may develop behind an obstructing bronchial tumor. Aspiration pneumonia may occur in those with esophageal tumor either secondary to an obstruction or as a result of a tracheo-esophageal fistula.
The gastrointestinal system is the most common site of indigent organisms causing infection in patients with neutropenia. Clostridium difficile infection may present with fever and diarrhea and must be considered in those who are already taking antibiotics. Fungal and viral infections of the esophagus need to be suspected in those with dysphagia and odynophagia. Anaerobic infections may be a factor in severe mucositis or gingivitis and in those patients with perianal discomfort. Spontaneous bacterial peritonitis may be a cause of fever in patients with ascites. A urinary catheter increases the risk of urinary tract infection as does the presence of urinary obstruction, but the presence of asymptomatic bacteriuria in a patient with non-neutropenic cancer is not usually an indication for antibiotic treatment.
Central nervous system infections can be difficult to diagnose and usually require lumbar puncture to confirm. Infection is the most common complication of Ommaya reservoirs, used to administer intraventricular chemotherapy, and is more likely to occur in those with previous radiotherapy or in whom repeated surgical procedures have been necessary (21). Most infections are due to Staphylococcus epidermidis and can usually be successfully treated with antibiotics (22).
Particular attention to sites of recent surgery is essential in assessing infection. Surgical collections may include infected hematomas that develop following surgery. The skin is also a common site of infection that may range from infected decubitus ulcers to herpes zoster infections. The use of percutaneous catheters in oncology has created another portal for the introduction of infection in patients with cancer. Of a total of 322 indwelling devices placed in 274 patients with cancer by a single surgeon, device-related sepsis occurred in 28 of 209 patients (13%) with catheters and 6 of 113 patients (5%) with subcutaneous ports (23). Triple lumen catheters were associated with a higher rate of thrombosis but not of infection. The complications of 1630 venous access devices for long-term use in 1431 consecutive patients with cancer were reviewed (24). Of the catheters inserted, 341 of 788 (43%) had at least one device-related infection compared with 57 of 680 (8%) of the completely implanted ports (p = .001). The number of infections per 1000 device days was 2.77 for catheters compared with 0.21 for ports (p = .001). The predominant organisms isolated in catheter-related bacteremia were gram-negative bacilli (55%) compared with gram-positive cocci (65.5%) in port-related bacteremia. Patients with solid tumors were less likely to have device-related infectious morbidity compared with patients with hematologic cancers.
Transfusion-Related
Blood products, administered extensively to patients with cancer, may be associated with febrile reactions. The incidence of side effects following the administration of over 100,000 units of red blood cells to more than 25,000 patients with cancer over a 4-year period was retrospectively reviewed (25). Of all transfused units, 0.3% had a transfusion-associated reaction; 51.3% were febrile nonhemolytic, and 36.7% were allergic urticarial reactions. Only 17 hemolytic reactions (4 immediate, 13 delayed) were documented. The incidence of transfusion-related side effects was significantly lower in this study than that reported in the noncancer population. Infection may also be a source of fever in patients receiving blood products. The Canadian Red Cross (26) estimated that the true positive rate of bacterial contamination of platelet concentrate units was between 4.4 and 10.7 per 10,000 units and recommended screening of all such units. The percentage of those patients developing bacteremia or septicemia from infected units was not discussed.
Thrombosis
Trousseau’s self diagnosis of gastric cancer on the basis of venous thrombosis is a reminder that patients with cancer are at particular risk of thrombosis. Deep-vein thrombosis may present with fever and, given the uncertainty of clinical diagnosis, investigation may be necessary in the “at risk” patient. Pelvic thrombophlebitis may sometimes occur after pelvic surgery and, if septic, may manifest with either low- or high-grade fever. Pulmonary embolus also needs to be considered in the differential diagnosis of fever in patients with cancer. Of 97
patients with confirmed pulmonary embolus in the Urokinase PE Trial, 17 had associated malignancy (27). Of those with confirmed malignancy, 54% had a fever >37.5°C and 19.6% had a fever >38°C. Forty-one percent of the patients with cancer had associated sweating. Pulmonary infarction, with the primary signs of tachypnea, tachycardia, and fever, may be a presentation of pulmonary thrombi. Other thrombotic syndromes, such as cerebral venous thrombosis, although associated with fever, are not common in the setting of malignancy.
patients with confirmed pulmonary embolus in the Urokinase PE Trial, 17 had associated malignancy (27). Of those with confirmed malignancy, 54% had a fever >37.5°C and 19.6% had a fever >38°C. Forty-one percent of the patients with cancer had associated sweating. Pulmonary infarction, with the primary signs of tachypnea, tachycardia, and fever, may be a presentation of pulmonary thrombi. Other thrombotic syndromes, such as cerebral venous thrombosis, although associated with fever, are not common in the setting of malignancy.
Hemorrhage
Gastrointestinal bleeding may present with fever and should be considered in the differential diagnosis of a patient with low-grade fever and sweats. However, in a major review of fever in patients with cancer (10), serious hemorrhage was followed by fever in a minority of cases; the usual sequence has been that of hemorrhage in an already febrile patient.
Table 9.4 Cytotoxic Agents Associated With Fever After Administration | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Drugs
Drug-associated fever is an ill-defined syndrome in which fever is the predominant manifestation of an adverse drug reaction. It is normally a diagnosis of exclusion (28). The drugs commonly associated with fever are antibiotics, cardiovascular drugs, central nervous system drugs (e.g., phenytoin), cytotoxics, and immune therapy drugs (either as biologic response modifiers or growth factors). Antimicrobial agents were responsible for 46 of 148 drug-related fevers in a review of the experience of two hospitals in Texas and the United Kingdom with a mean lag time from initiation of treatment to onset of fever of 21 days (median, 8 days). For 11 cases of cytotoxic-induced fever, the mean lag time was 6.0 days with a median of 0.5 days. Shaking chills were more common with the administration of cytotoxic-associated fever than with other drugs (29).
Cytotoxics
There is a diverse range of cytotoxic drugs whose administration is associated with fever (Table 9.4). The febrile response to bleomycin was described in the original phase 1 studies (30) and characteristically occurs 3–5 hours after injection. It is more common after intravenous than after intramuscular injection. It is seen in approximately 25% of patients who were administered the drug. The fever becomes less frequent with repeated injections. An anaphylactic reaction manifested by hyperpyrexia, shock, hypotension, urticaria, and wheezing occurs in 1% of patients administered bleomycin (31). Fever following the administration of cisplatin was also reported in early clinical trials (32). Streptozocin administration may result in fever associated with chills, as can cytarabine and etoposide. Fever can occur after the administration of 5-fluorouracil and high-dose methotrexate. Confusion concerning the etiology of a fever arises more commonly in the situation of intensive chemotherapeutic regimens where patients with neutropenia may be administered cytotoxic agents that cause fever. Awareness of the symptoms produced by the different agents assists in discerning the etiology of the fever.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree