BACKGROUND
Historically, pregnancy in women with diabetes is associated with high perinatal morbidity and mortality. The fetal and neonatal complications include increased risk of congenital malformations, miscarriage, preterm delivery, stillbirth, accelerated fetal growth with its attendant risk of traumatic vaginal delivery and shoulder dystocia, cardiomyopathy, respiratory distress syndrome, and neonatal metabolic derangements, especially hypoglycemia. Fetuses of women with vascular complications of long-standing diabetes, notably diabetic nephropathy with or without accompanying hypertension, are also at risk of intrauterine growth restriction (IUGR) as a result of placental insufficiency. Many of the late fetal and neonatal complications in diabetic pregnancies can be explained by the Pedersen hypothesis.1 This states that maternal hyperglycemia resulting in fetal hyperglycemia causes marked fetal hyperinsulinemia via fetal pancreatic beta cell overstimulation. This in turn causes accelerated fetal growth, excess subcutaneous fat, and increased hepatic glycogen storage, features often referred to as macrosomia and all of which can contribute to traumatic delivery and neonatal metabolic derangements. This theory has driven the clinical management of diabetes in pregnancy, with the belief that better glycemic control in the mother can reduce perinatal morbidity and mortality. This chapter focuses on the surveillance required in the third trimester in order to minimize perinatal mortality and morbidity in structurally normal fetuses.
The 2002–2003 UK Confidential Enquiry into Maternal and Child Health (CEMACH)2 showed that babies of women with Type 1 or Type 2 diabetes are five times more likely to be stillborn and three times more likely to die in their first month of life compared with those of mothers without diabetes. The perinatal mortality rate was 31.8 per 1000 births, compared to the national rate of 8.5 per 1000 births. Approximately 80% of these losses were stillbirths, 80% of these babies being structurally normal. Perinatal mortality in European countries and other UK regional studies is comparable, and ranges from 27.8 to 48 per 1000 births.
The goal of obstetric surveillance is to identify fetuses at risk, in order to intervene in a timely and appropriate fashion and reduce perinatal morbidity and mortality. However, the CEMACH enquiry3 during 2002–2003 found that fetal surveillance was poor in 20% of 37 pregnancies with antenatal evidence of fetal growth restriction and in 45% of 129 pregnancies with antenatal evidence of accelerated growth; the enquiry did not explain what the deficiencies were nor make recommendations regarding optimal surveillance.
PATHOPHYSIOLOGY OF FETAL COMPROMISE IN DIABETES IN PREGNANCY
Before choosing a test to assess fetal well-being, it is important to understand the pathophysiology of fetal compromise in diabetes in pregnancy. When fetal death occurs, it is usually in the final weeks of pregnancy in the context of poor glycemic control, polyhydramnios, and/or accelerated fetal growth.4,5 In contrast, diabetic women with vasculopathy and/or pre-eclampsia may develop IUGR and fetal demise as early as the second trimester, probably related to placental vascular disease.
The pathophysiology of fetal compromise in diabetic pregnancy where the fetus is normally grown or large for dates is likely to be multifactorial. It is probable that the majority of unexplained stillbirths result from chronic fetal hypoxia and/or fetal acidemia secondary to maternal/fetal hyperglycemia and fetal hyperinsulinemia.
Evidence of fetal hypoxia is seen in the form of raised fetal erythropoietin (EPO) levels in infants with intrauterine growth acceleration.6 Extramedullary hematopoiesis is found more often in stillborn infants of mothers with diabetes.7 Thickening of the basement membrane of chorionic villi has been described in placentae of pregnancies in women with diabetes,8 which potentially could reduce oxygen transfer.
Maternal hemoglobin A1c (HbA1c) concentrations in the third trimester correlate with fetal umbilical venous EPO at delivery9 suggesting that antepartum control of maternal hyperglycemia is a significant factor associated with fetal hypoxia. A recent systematic review of four studies of adverse pregnancy outcome in women who have Types 1 and 2 diabetes found increased perinatal mortality associated with poor glycemic control (pooled OR 3.23, 95% CI 1.87 – 4.92),10 although the studies reviewed had methodologic limitations. Marked oscillations in maternal glycemic control may explain accelerated fetal growth and fetal compromise seen in some pregnancies with apparently excellent diabetic control.11 Maternal hypoglycemia does not seem to impact the fetus significantly; however, there are few studies addressing this issue.
Hyperglycemia and hyperinsulinemia in fetal lambs increase oxygen consumption with a simultaneous decrease in oxygen content.12 Hyperglycemia increases fetal glycosylated hemoglobin, which shifts the fetal oxygen dissociation curve, thus reducing red cell oxygen delivery at tissue level.13 In late pregnancy, the switch from HbF to HbA production is delayed in women with diabetes, and this may further reduce tissue oxygen availability. Fetal amniotic fluid insulin levels correlate significantly with fetal plasma EPO levels independently of maternal glycemia, suggesting that insulin exerts an effect on fetal oxygenation beyond that of maternal and fetal glycemia.9 Fetal hyperinsulinemia may also result in hypokalemia in the fetus (possibly predisposing to fatal cardiac arrhythmia). There is a significant association between fetal plasma insulin levels and the degree of fetal acidemia,14 and significant acidemia has been observed even in the absence of hypoxia.15 Glucose oxidation and oxygen consumption are increased by hyperinsulinism, this effect being independent of that caused by hyperglycemia. The reduced capacity of the fetus for oxidative metabolism and low pyruvate dehydrogenase activity result in hyperlactinemia. Diabetic ketoacidosis is dangerous for both the mother and the fetus, the risks to the latter probably being related to acidosis (see chapter 19).
ACCELERATED FETAL GROWTH
Various definitions of accelerated fetal growth are in use, such as birthweight over 4000 g, over the 90th centile, or two standard deviations above the mean weight for gestation corrected for sex. The last mentioned is preferred, but even this does not characterize the selective organomegaly seen in the infant of the woman with diabetes. The CEMACH report2 found that 21% of singleton babies of women with diabetes weighed over 4000 g compared with 11% in the general population, and over 1% of babies of women with diabetes weighed under 1000 g compared with 0.5% in the general population.
A strong relationship between fetal weight and umbilical plasma insulin,16 and a direct relationship with amniotic fluid EPO levels, have been observed, suggesting that the larger the fetus is, the greater the risk of fetal hypoxia. Growth acceleration may start as early as 18 weeks of gestation.17 However, the growth potential of fetuses seems to be determined by prevailing maternal glycemia before then and excessive growth can continue despite optimum glycemic control subsequently.18 Nonetheless, optimal glycemic control during pregnancy is associated with a reduced incidence of accelerated fetal growth19 and therefore with improved perinatal outcome.
Accelerated fetal growth is associated with a significant increase in the risk of fetal death in the presence but not the absence of maternal diabetes. Seeds et al reported an OR of 3.4 (95% CI 1.1–9.6; p < 0.01) for intrauterine death at or beyond 24 weeks of gestation for a birthweight over the 95th centile and an OR of 3.2 (CI 1.3–7.4; p < 0.01) for a birthweight between the 90th and 95th centiles compared with birthweights between the 25th and 75th centiles.20
One of the major challenges relates to the best way to minimize the likelihood of shoulder dystocia, brachial plexus injury, and other major birth trauma in babies with scan-detected accelerated growth. The UK CEMACH2 report into pregnancies in women with pregestational diabetes found an incidence of Erb palsy of 4.5 per 1000 births, 10-fold greater than for the general population, but 25% of babies with Erb palsy born to women with diabetes were delivered by cesarean section. It also confirmed that shoulder dystocia is more common in larger babies, ranging from 1% in babies less than 2500 g to 43% in babies over 4500 g. In addition, it has been reported that babies of women with diabetes have a three-to seven-fold greater risk for shoulder dystocia at each given weight category compared with non-diabetic women.21 This can be explained by anthropometric differences between babies of diabetic and non-diabetic mothers.22 While both detection of accelerated fetal growth (see below) and prediction of shoulder dystocia, which relates to more than fetal size alone, are poor, studies using ultrasound weight prediction followed by cesarean for those cases where birthweight is predicted to be more than 4250 g have shown a reduction in shoulder dystocia, but at the expense of an increase in cesarean section rate (see also chapter 20). In addition, many babies estimated by scan to be large have actual birth-weights less than 4250 g:23 443 cesarean sections with a scan-estimated fetal weight (EFW) threshold of 4500 g would need to be performed to prevent one permanent brachial plexus injury, or 489 with an EFW threshold of 4000g.24
FETAL SURVEILLANCE METHODS
Surveillance includes monitoring of fetal growth and assessment of fetal well-being. Given the multifactorial nature of the etiology and the timing of fetal demise in pregnancies with diabetes, it is difficult to know which forms of monitoring, if any, are appropriate. It is generally considered that standard clinical assessment needs to be supplemented by other methods of surveillance, although two recent reviews of fetal monitoring in diabetic pregnancy7,25 show that no currently available technique has been proven to predict the fetuses at risk or to prevent poor outcome. This is not surprising as the methods assessed were not designed to predict the pathologic processes likely to precede fetal demise. Accordingly, some clinicians reserve surveillance only for those pregnancies with additional issues, such as vasculopathy, hypertension or growth restriction, that have more predictable fetal complications and better-established modes of surveillance.
The tools available for fetal surveillance include:
- Antenatal CTG
- Ultrasound assessment of fetal growth
- Ultrasound assessment of amniotic fluid volume
- Biophysical profile
- Umbilical artery Doppler velocimetry
- Amniocentesis – assessment of fetal lung maturity, fetal insulin, EPO
- Magnetic resonance imaging (MRI) spectroscopy.
Antenatal cardiotocography
Antenatal CTG records fetal heart rate and displays it in relation to spontaneous uterine activity, a technique sometimes referred to as a non-stress test (NST). Antenatal contraction stress tests, in which the fetal heart rate is observed during uterine activity induced by, for example, an infusion of oxytocin, are rarely used.
Outside of diabetes, antenatal CTG is used to detect antenatal fetal compromise in high-risk pregnancies. A Cochrane Systematic Review however revealed only four published studies including a total of 1588 high-risk pregnancies, only one of which pertained to diabetes.26 These failed to show a significant effect of antenatal CTGs on perinatal morbidity or mortality. There are no randomized controlled trials assessing the value of antenatal CTG for fetal surveillance in diabetes in pregnancy. Non-randomized studies indicate that the tool is a poor predictor of fetal compromise in diabetes with fetal demise reported hours after a normal trace.27 This is not surprising considering the probable pathogenesis of fetal demise in diabetes in pregnancy.
Barrett et al reviewed seven studies of antepartum CTGs for fetal surveillance and found that within 7 days of a normal CTG there was a stillbirth rate of 1.4% in pregnancies with diabetes, similar to the figure for pregnancies complicated by IUGR (2%); there were no stillbirths in pregnancies with other risk factors, such as being postdates or pre-eclampsia.28 Attempts to correlate CTG patterns with maternal glycemic status have been unsuccessful.29,30 Tincello et al29 found that 10.7% of computerized CTG recordings in pregnancies complicated by Type 1 diabetes had no episodes of high variation (defined as long-term variation exceeding a gestation-dependent threshold, with the presence of high variation considered to be a sensitive indicator of fetal well-being in normal pregnancy), compared with the expected value of 0.8% in uncomplicated singleton pregnancies, a difference of 9.5% (95% CI 4.5–15.3). The authors concluded that along with other differences in short-term variation, basal heart rate, frequency of fetal movements, and heart rate accelerations, these changes may represent a delay in fetal maturation. They did not identify any relationship between these changes and adverse fetal outcome, and therefore computerized CTG must be used with caution in women with diabetes.
In conclusion, available evidence does not support the routine use of antenatal CTG in pregnancies with diabetes outside the usual indications, including reduced fetal movements, fetal growth restriction or antepartum hemorrhage.
Ultrasound estimation of fetal growth
There is no clear consensus that monitoring fetal size by ultrasound in pregnancies with diabetes without risk factors for IUGR is beneficial31 or necessary. The UK National Institute for Health and Clinical Excellence (NICE) guidelines32 state that three growth scans, including estimation of amniotic fluid index, should be carried out at 28, 32, and 36 weeks of gestation, but no guidance is offered as to how the information obtained should subsequently be used.
Conventional ultrasound biometry is based on predicting IUGR, and is less accurate for detecting large babies, including those babies whose mothers have diabetes:33 it overlooks 10–20% of babies with accelerated growth.31 This is an important deficiency as diabetes influences the abdominal circumference (AC) but not bony measurements via its effect on insulin-sensitive tissues such as the liver (glycogen storage) and abdominal wall adipose tissue (Fig. 12.1). Accordingly, ultrasound measurements made to detect accelerated fetal growth are unreliable and therefore cannot be expected to predict trauma at delivery for these babies;22 as such they are of debatable value.
Fig. 12.1 Fetal ultrasound growth charts. Normal growth of head and femur with accelerated growth of abdominal circumference.
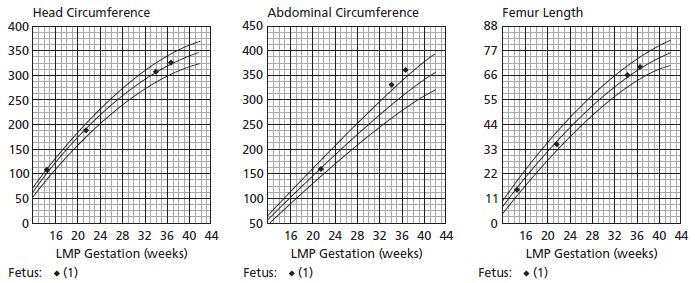
A systematic review31 of 63 studies including a total of 19 117 women concluded that there was no difference in accuracy between ultrasonographic EFW and AC in the prediction of birthweight over 4000 g. These studies were in women without diabetes and therefore cannot be extrapolated directly to pregnancies in women with diabetes, but it is likely that accuracy would be even lower in the latter. In two studies of pregnant women with diabetes, the sensitivity of ultrasound for predicting fetal weight over 4000 g was relatively poor, ranging from 33% to 83%, and specificity from 77% to 98%.34 The correlation between ultrasound determination of fetal size or fetal growth and fetal hyperinsulinemia (measured using amniotic fluid insulin levels) is also poor, and therefore ultrasound is not likely to be able to predict those babies at risk of the consequences of fetal hyperinsulinemia, such as intrauterine death and neonatal hypoglycemia.35 In addition, growth continues to accelerate late into the third trimester, thereby further reducing the accuracy and usefulness of scanning as delivery approaches.36
Three-dimensional (3D) ultrasound volumetric assessments of fetal weight within 7 days of delivery has been shown to be superior to conventional two-dimensional (2D) biometry in a cross-sectional study in women without diabetes.37 Fetal liver volume may also be assessed using 3D ultrasound. In a cross-sectional study, fetal liver volume was significantly greater in fetuses of 32 women with diabetes compared with matched controls, even after normalization for EFW. Fetal liver volume also correlated significantly with glycosylated hemoglobin.38 More research is needed in this area before incorporation into routine clinical practice can be recommended.
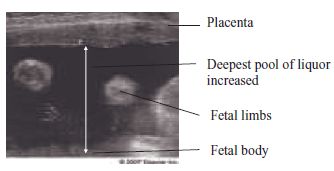
Fig. 12.2 Polyhydramnios on ultrasound scan.