Fertility preservation options
Standard strategy
Ovarian stimulation required
Invasive procedure required
Male partner required
Risk of malignant cells contamination
Delay in cancer treatment
Available in all centers
Oocyte cryopreservation
+
+
+
–
–
+
–
Embryo cryopreservation
+
+
+
+
–
+
–
Ovarian suppression with GnRH analogs
–
–
–
–
–
–
+
Ovarian tissue cryopreservation
–
–
++
–
+
–
–
4.2 Mature Oocyte and Embryo Cryopreservation
Controlled ovarian hyperstimulation (COH) with gonadotropins is needed to obtain more than one oocyte and is a key component in the success of in vitro fertilization (IVF), as well as in cycles aiming to preserve fertility by oocyte or embryo cryostorage [3]. The choice of the specific COH protocol is generally based on the preferences of each IVF unit and is influenced by the time available before the initiation of radio-/chemotherapy. Although multiple different COH protocols have been proposed, the majority of patients are treated with a GnRH antagonist short protocol, which allows the shortest deferral of the initiation of chemotherapy [4].
Conventionally, ovarian stimulation with GnRH antagonists can be started either in the early follicular phase or in the luteal phase [4]. The first approach requires awaiting menses: gonadotropin stimulation begins on day 2–3 of the cycle, while GnRH antagonist is usually started on day 6, when the size of the leading follicle reaches 12–14 mm [5]. Administration of a GnRH antagonist (e.g., 3 mg cetrorelix subcutaneously) in the luteal phase, instead, induces corpus luteum breakdown and menstruation ensues a few days later [6, 7]. Ovarian stimulation can therefore be initiated quickly and the GnRH antagonist would be restarted later to prevent premature LH surge [7].
Recently, the introduction in the clinical practice of the so-called “random-start” ovarian stimulation protocol has provided a further decrease of the total time required for ovarian stimulation [4]. This novel technique is supported by the demonstration of a series of three major follicle-recruiting waves during a normal menstrual cycle, allowing to start follicular growth irrespective of the cycle phase [3]. In fact, in the “random-start” protocol, COH can be initiated either in the late follicular phase or in the luteal phase, following spontaneous LH surge or after ovulation induction with human chorionic gonadotrophin (hCG) or a GnRH agonist (Fig. 4.1) [3]. Both these ovarian stimulation strategies are as effective as conventional start protocols [8], challenging the traditional concept that antral follicles observed in the luteal phase have undergone atresia and are useless [8, 9].
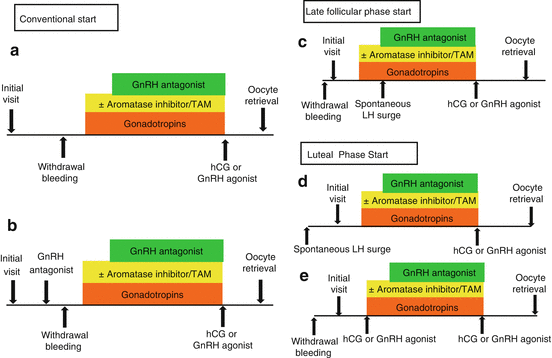

Fig. 4.1
Conventional and random-start COH. (a) Conventional start in early follicular phase, (b) conventional start in luteal phase after luteolysis induced by GnRH antagonist, (c) random start in late follicular phase, (d) random start in luteal phase following spontaneous LH surge or after ovulation induction with hCG or GnRH agonist (e)
During COH there is a potential risk that the supra-physiologic estradiol (E2) levels could promote the growth of estrogen receptor-positive (ER+) breast cancer cells [10]. The rise in E2 is directly proportional to the number of growing follicles; for this reason, alternative and potentially safer protocols have been introduced for these patients, including natural cycle IVF, stimulation protocols with tamoxifen (TAM) alone or combined with gonadotropins, and stimulation protocols with aromatase inhibitors (AI) [11].
Natural cycle IVF does not allow to obtain more than one oocyte or embryo per cycle and has a high rate of cycle cancellation due to precocious follicle rupture. This strategy may thus result ineffective when chemotherapy is imminent and the patient does not have a chance for a second attempt [11].
TAM is a non-steroidal compound related to clomiphene, as effective as clomiphene for COH in anovulatory patients [12]. TAM is a selective ER modulator (SERM) with antioestrogenic actions on breast tissue leading to inhibition of the growth of breast tumors due to competitive antagonism of E2 at its receptor site [12]. TAM can be used for ovulation induction starting on day 2–5 of the menstrual cycle in doses of 20–60 mg/day; it may be used alone or in combination with gonadotropins [13]. Ovulation induction with TAM for fertility preservation in cancer patients has been shown to increase mature oocyte and embryo yield compared with natural cycle IVF, reducing cycle cancellation rate [13]. The combination of low-dose FSH with TAM further increased the number of cryopreserved oocytes and embryos compared with ovulation induction with TAM alone [14]. Although E2 levels during COH with TAM are not very low, its use in ER+ breast cancer patients is protective, due to its antiestrogenic effect on breast tissue [13]. The safety of TAM coadministration during COH has been recently confirmed by the assessment of persistently high serum E2 levels in premenopausal breast cancer patients safely treated with adjuvant TAM, at up to 6 years follow-up [15].
AI, such as letrozole, markedly suppress plasma E2 levels by competitively inhibiting the activity of the enzyme aromatase [16]. Aromatase is a cytochrome P450 enzyme complex that catalyzes the conversion of androstenedione and testosterone to estrone and E2, respectively [17]. Centrally, AI release the hypothalamic-pituitary axis from the estrogenic negative feedback, increase the pituitary secretion of FSH, stimulate follicle growth, and, thereby, can be used for ovulation induction [18]. The use of letrozole alone for ovarian stimulation has been associated with lower E2 levels than those observed in a natural cycle [19]. Oktay tested letrozole in association with gonadotropins and a GnRH antagonist (“COST-LESS” protocol) [14]: letrozole was started orally from day 2 or 3 of the cycle at a dose of 5 mg day, while gonadotropins (150–300 UI) were started 2 days later. A GnRH antagonist was added when E2 levels exceeded 250 pg/ml or when the leading follicle reached 14 mm diameter in order to prevent premature LH surge. All medications were discontinued the day of hCG trigger, and letrozole was reinitiated after oocyte retrieval and continued until E2 levels fell to 550 pg/ml. The final maturity of oocytes was triggered with hCG, that, however, potentiates the endogenous production of E2 during the luteal phase, may cause ovarian hyperstimulation syndrome (OHSS) and can cause a false-positive pregnancy test, creating confusion when a pregnancy test is performed prior to initiation of chemotherapy [10]. In order to circumvent these problems, hCG trigger was later replaced by GnRH agonist trigger (1 mg leuprolide acetate), leading to significantly faster drop in E2 levels, significantly lower rate of moderate/severe OHSS and comparable number of mature oocytes [20]. Compared to a conventional IVF protocol, the COST-LESS protocol resulted in a significantly lower peak estradiol levels and in a 44 % reduction in gonadotropin requirement, while the length of stimulation, the number of embryos obtained, and the fertilization rate were similar [14].
Stimulation protocols using letrozole are currently preferred over TAM protocols for patients with ER+ breast cancer who need fertility preservation because it allows to minimize the risk of high E2 exposure [14] and of cancer recurrence [21].
Maximizing the number of embryos or oocytes cryopreserved during a fertility preservation cycle is extremely important to increase the chance of future pregnancies: cancer patients have shortage of time and often a single COH cycle to try [22]. Traditionally, breast cancer patients have time to undergo one cycle of COH before initiating adjuvant chemotherapy, which typically occurs after breast surgery [3]. In the event of a poor response, multiple cycles are often not feasible owing to time constraints. Unfortunately in cancer patients, both the specific malignancy and the patient’s general conditions may have a negative impact on the response to ovarian stimulation [23]. In breast cancer, some authors [24] reported that in BRCA1 mutation-positive patients a low response to ovarian stimulation occurred significantly more frequently than in patients with BRCA2 or without BRCA mutation. BRCA genes play an essential role in double-strand DNA break repair, and their mutations are associated with an increased risk of breast and ovarian cancers [24]. In patients with BRCA mutations, oocytes may be more prone to DNA damage, clinically manifesting as diminished ovarian reserve, poor ovarian response to COH, or earlier menopause [25].
One of the most obvious strategies to increase the embryo and oocyte yield could be the use of higher doses of gonadotropins [3]; surprisingly, a recent evidence suggests that a high-dose FSH stimulation does not improve pregnancy outcomes, and may be associated with a lower live birth rate [26], supporting the theory that high doses of FSH might stimulate the recruitment of chromosomally abnormal or incompetent oocytes [27]. Another strategy to improve oocyte yield is the early referral (before breast surgery) that allows breast cancer patients to undergo multiple COH cycles without delaying the initiation of adjuvant chemotherapy [28].
4.3 Ovarian Tissue Cryopreservation
Ovarian tissue cryopreservation (OTC) is the main option available to preserve fertility in women under the age of 38 years who require urgent cancer treatments, such as neo-adjuvant chemotherapy. OTC does not need ovarian stimulation and is independent from menstrual cycle: it can be performed in a few days, without any delay in the beginning of chemotherapy.
OTC allows to retrieve and cryostore a great number of primordial follicles that are relatively resistant to cryodamage [29]. Moreover, this technique permits to restore endocrine function after reimplantation of ovarian tissue and is the only option for prepubertal patients. The main disadvantage of the strategy is the need of invasive procedures both for tissue harvesting and transplantation. Of note, OTC may cause a further decrease of ovarian reserve as an effect of ovarian surgery. Another crucial point is the risk of reintroducing malignant cells when transplantation is performed.
It has not yet been established whether ovarian biopsy or unilateral oophorectomy should be preferable to retrieve ovarian tissue. Indeed patients who underwent unilateral oophorectomy were reported to have a significant number of spontaneous pregnancies; however, the removal of an entire ovary might be too aggressive, and could reduce the ovarian reserve too much [30]. Several studies concluded that laparoscopy should be considered the gold standard for ovarian tissue harvesting, although ovarian tissue can be obtained during contingent laparotomic surgeries when these are needed [31]. When ovarian tissue has been recovered, transport on ice from the place of removal to the laboratory can last up to 20 h, thus allowing the creation of few specialized centers where cryopreservation procedure takes place [32]. In the laboratory ovarian cortex is enucleated from the medulla and cut in small fragments. One or two fragments are usually sent to histology. Ex vivo retrieval of mature or immature oocytes and their vitrification (after in vitro maturation in case of immature oocytes) is feasible and improves the efficiency of fertility preservation programs [33]. Finally the ovarian tissue is stored in liquid nitrogen after the freezing procedure. Although slow freezing of ovarian cortex is still applied in most fertility preservation laboratories and has resulted in most of the live births after transplantation, vitrification of ovarian tissue is an emerging focus of investigation, and the first live birth after transplantation of vitrified-warmed tissue was recently reported [34, 35].
To date orthotopic or heterotopic transplantation is the only available option to restore fertility using cryopreserved ovarian tissue, as other techniques require additional research before becoming available for humans.
The transplant is usually performed when the patient is willing to get pregnant, with the permission of the oncologists, as the duration of ovarian tissue transplanted is limited in time [36]. For the same reason, not all the fragments of ovarian cortex available are thawed and transplanted at the same time, when feasible.
Transplantation can take place either into the pelvic cavity, in the orthotopic transplant, or in alternative sites in the heterotopic transplant (Table 4.2). In consideration of the low invasivity, the subcutaneous site is sometimes associated with transplantation at the orthotopic site [37, 38].
Table 4.2
Orthotopic versus heterotopic ovarian transplantation
Heterotopic transplantation | Orthotopic transplantation | |
|---|---|---|
Advantages | Easy transplantation procedure Easy access for follicular monitoring and oocyte collection | Possibility of natural conception Restoration of fertility widely demonstrated Favorable environment for follicular development |
Disadvantages | Restoration of fertility demonstrated only in one case IVF procedure required Effect of the local environment on oocyte quality unknown | Invasive transplantation procedure |
There are essentially two techniques of orthotopic (in the pelvis) transplantation that may be used depending on the presence or not of at least one remaining ovary. If at least one ovary is present, the technique starts with decortication of the ovary in order to have access to the medulla and its vascular network. Ovarian cortical pieces are then fixed to or placed on the medulla. If ovaries were previously removed, a peritoneal pocket to place ovarian fragments may be created. Transplantation can be performed at the peritoneal site even if a non-functioning ovary is still in place, in addition to the transplant at the ovarian site or as unique location [39]. Some authors performed transplantation at the ovarian site [40–42], other groups used the peritoneal window [32, 43–46], and lastly there were some associating the two techniques [47–53]. Transplantation can be performed either using laparotomy [40–42, 44], laparoscopy [32, 43, 45–50, 52], or a combination of the two techniques [51], while some authors suggest the possible use of robotic surgery [54].
Even if ovarian tissue is amenable to avascular transplantation the potential of revascularization of the graft is the most important factor for success, because it establishes the survival rate of the follicle pool within the graft. As for the prevention of ischemic damage, Donnez proposed the “two-step” approach. During the preparatory laparoscopy (7 days before reimplantation), he created a peritoneal window with the goal of inducing angiogenesis and neo-vascularization in the area, by triggering endogenous processes of new vessel formation. During the second intervention, he reimplanted the frozen–thawed ovarian fragments where a newly formed vascular network in the peritoneal window was clearly seen [47]. Demeestere suggested to associate at the “two-step” technique a subcutaneous heterotopic transplantation at the abdominal site; Piver and Roux suggested to add small pieces 1–2 mm of thawed ovarian cortex during the first surgery to facilitate the production of angiogenic factors [48, 50]. As an alternative, Revel proposed the use of microorgans (MOs), fragments whose thickness are about 300–350 mM, that remain viable and transcribe specific genes for long periods both in culture and when implanted into hosts [44]. Callejo proposed to use angiogenic factors to improve the vascularization of the implant and its quality; he used a gel preparation of PRP (plasma rich in platelets) to impregnate the thawed cubes of ovarian tissue and to fill the peritoneal pockets where the fragments were placed [45].
The risk of reintroducing malignant cells theoretically exists in breast cancer patients [43]. Breast cancer can metastasize to the ovaries, more commonly in advanced-stage cancer, even if the development of an ovarian tumor is more likely to be of primary ovarian origin than a breast cancer metastasis [55]. A special attention should be reserved to BRCA mutation carriers.
Different studies based on the examination of cryopreserved ovarian tissue from women with breast cancer using both conventional histology and immunohistochemistry revealed no evidence of malignant cell involvement [56–58].
On the other hand, in a large review of 5,571 female autopsies, Kyono evidenced ovarian metastases in 24.2 % of breast cancer patients [59]. As these data were obtained as results of autopsies, they reflect the risk of ovarian involvement in patients with advanced breast cancer; patients who are offered ovarian tissue cryopreservation have a minimal risk of dissemination and ovarian involvement. Anyway, the results from this study suggest that a great caution is necessary when transplanting the tissue of breast cancer patients. A pilot study by Donnez demonstrated that cryopreserved ovarian tissue from patients with advanced-stage breast cancer may contain cells expressing the MGB2 gene, even if the real malignant potential of these cells is not yet known [60]. Ernst reported a legal termination of pregnancy due to breast cancer recurrence in a patient who spontaneously conceived after ovarian tissue transplant, even though the authors considered unlikely that the transplanted tissue had any effect on the recurrence of cancer [61]. Apart from the possibility of tumor contamination of the cryopreserved tissue, the return of natural ovarian function may have an impact on the course of breast cancer.
For patients with a potential risk of having malignant cells in their cryopreserved ovarian tissue, other options could be the follicle culture with in vitro maturation [33, 62], the grafting of isolated follicles [63], ovarian tissue purging to eliminate malignant cells [64], and artificial ovaries composed of primordial follicles combined with disease-free stromal elements or placed in an alginate matrigel matrix [65].
The analysis of the recovery of ovarian function after ovarian tissue transplantation is difficult because of the lack of reports in the literature which indicate how many patients in the world have been subjected to the procedure. Anyway the regain of endocrine function has been described in all published cases of ovarian transplantation, both orthotopic and heterotopic. Ovarian function has been demonstrated to persist up to 7 years after transplantation with a mean duration of 4–5 years [36].
To date almost 30 live births have been reported worldwide after orthotopic ovarian transplant [39–42, 44–53, 66–68], whereas heterotopic graft has led to one twin pregnancy [69], a biochemical pregnancy [38], and four spontaneous pregnancies with three live births as a result of a reactivation of the native ovary [70]. Most pregnancies were obtained from women younger than 30 years at the time of cryopreservation, as the age at ovarian retrieval is one of the most important predictive factors, since the follicular reserve is age dependent. After orthotopic transplant, more than 50 % of women were able to conceive naturally and this fact constitutes a good point in favor of orthotopic reimplantation. Pregnancy outcomes were similar to those in the general population and all the babies were healthy.
In conclusion, the effectiveness of ovarian tissue cryopreservation and transplantation in terms of endocrine function and fertility restoration has been proven, and even if still experimental, OTC is a good option to preserve fertility in breast cancer patients when ovarian stimulation is not feasible.
4.4 Ovarian Suppression with GnRH Analogs
A GnRH analog (GnRHa) is a molecule derived from the native GnRH by substituting some of the amino acids.
GnRH agonists initially have a flare-up effect – stimulating the release of FSH and LH – while after chronic administration result in a downregulation of GnRH receptors and in a long-term desensitization of the pituitary cells producing gonadotropins. The final effect is decreasing FSH secretion and thus suppressing ovarian function, follicular development, and E2 secretion.
The rationale behind the use of GnRH agonists to reduce the gonadal toxicity of chemotherapy is based on the following issues:
Chemotherapy mostly affects tissues with a rapid cellular turnover: a state of inhibition during exposure to cytotoxic drugs may protect the ovaries.
Hypoestrogenism could imply a reduced ovarian perfusion and a lower dose of gonadotoxic agents reaching the ovaries.
Cyclophosphamide, and chemotherapy in general, alters the physiological quiescent status of the primordial follicles, inducing an increase in follicle activation, growth, and apoptosis. The derived damage to follicular ovarian reserve and the consequent reduction in estrogens, inhibin, and AMH cause an increase in FSH which further increases the accelerated recruitment of primordial follicles. Inhibiting FSH release by GnRH agonists can stop this vicious mechanism, otherwise called “ovarian reservoir burnout” [71]. Ovarian functional suppression through GnRH agonist administration has to be reached before the start of chemotherapy and should last during the entire period of cytotoxic treatment.
The advantages of this “medical” approach are the potential preservation of the overall ovarian function, the drug availability in every cancer care unit, and the fact that this method does not require an invasive procedure. Furthermore, it could be combined with other fertility preservation strategies with an expected improvement of fertility outcome. On the other hand, a complete onco-fertility counseling should explain to the patient the possibility of side effects related to the climacteric symptoms. An “add-back” therapy with estrogens, only through local formulations, could be considered in order to improve the overall quality of life and the therapeutic compliance.
A few data are available on the long-term efficacy of this strategy. The ASCO and the European Society for Medical Oncology (ESMO) guidelines still consider this strategy experimental [1, 72]. The potential protective effect of GnRH agonists for the prevention of chemotherapy-induced premature ovarian failure in breast cancer patients has been investigated in observational and phase II studies that showed an overall 91 % of reversibility. Several studies investigated the efficacy of GnRH agonists to preserve ovarian function in breast cancer patients candidates for chemotherapy; they were performed randomizing the patients to receive adjuvant and/or neo-adjuvant chemotherapy in combination with GnRH agonists vs. chemotherapy alone [73–80]. These studies reported conflicting results. Too many variables were involved and could bias the results: target population, type of chemotherapeutic medications, timing of therapies, patient’s age and prognosis, baseline ovarian reserve, concomitant subfertility conditions at time of diagnosis, duration of follow-up, definition of ovarian failure, etc. Aiming to overcome this study heterogeneity, the results of these studies have recently been reanalyzed in meta-analysis, and despite some controversies (Table 4.3) they showed a benefit of the administration of GnRH agonists in the prevention of chemotherapy-induced ovarian failure [81–88]. This benefit, however, concerned the resumption of menstrual bleeding and hormonal status, reflecting the steroidogenesis of the ovary. As for fertility, on the contrary, the efficacy of GnRH agonists’ administration remains unproven. For this reason, the recently published guidelines of ASCO and ESMO do not recommend this treatment for fertility preservation, but only as a strategy for hormonal ovarian function preservation [1, 72]. Hence, the GnRH analog strategy could be proposed once integrated in a wider scenario where the other fertility preservation options have to be offered.
Table 4.3




Meta-analysis about the use of GnRH analogs during chemotherapy to preserve gonadal function and fertility
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



