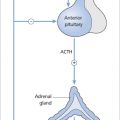
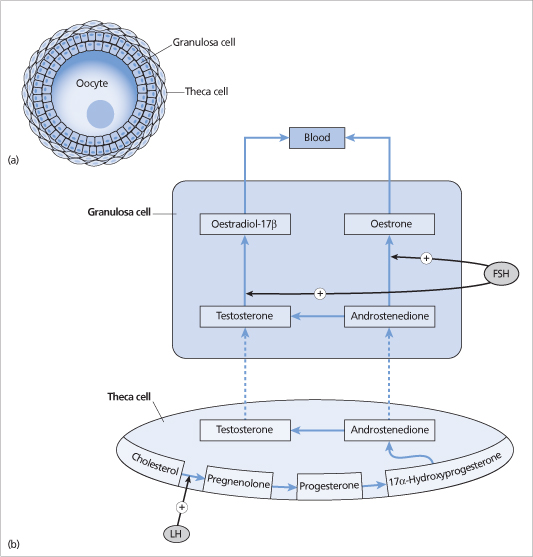
Figure 21.2 (a) Simplified diagram of an ovarian follicle consisting of an oocyte surrounded by granulosa cells and theca cells. (b) Androgens (testosterone and androstenedione) synthesized in the theca cells are converted to oestrogens (oestradiol-17 β and oestrone) in granulosa cells by the enzyme aromatase, which is stimulated by follicle-stimulating hormone (FSH). Luteinizing hormone (LH) stimulates the conversion of cholesterol to pregnenolone.
If, however, the oocyte becomes fertilized and is implanted in the endometrium, the early embryo begins to make chorionic gonadotrophin, which maintains the corpus luteum and progesterone production.
Endometrial changes
The endometrium undergoes marked alterations in response to the changing plasma levels of ovarian hormones. The rising serum oestradiol concentrations during the follicular phase of the menstrual cycle result in proliferation of the uterine endometrium and glandular growth. Hence the follicular phase is also known as the ‘proliferative’ phase.
After ovulation, increasing serum progesterone secreted by the corpus luteum plays an important role in converting the proliferative endometrium into a secretory lining. Hence the luteal phase is also known as the ‘secretory phase’.
As the corpus luteum function declines and the plasma oestrogen and progesterone levels decrease, the arterioles supplying the endometrium undergo vasospasm (caused by locally synthesized prostaglandins), causing ischaemic necrosis, endometrial desquamation and bleeding (the onset of menses at the beginning of the next cycle).
Oestrogens
The principal and most potent oestrogen secreted by the ovary is oestradiol. Oestrogens promote the development of secondary sexual characteristics (e.g. breast development), cause uterine growth and play an important role in the regulation of the menstrual cycle (see above).
Oestrogens act by binding to a nuclear receptor (either oestrogen receptor alpha or beta), which binds to specific DNA sequences and regulates the transcription of various genes. There is growing evidence that oestrogen receptors may also alter signal transduction by other mechanisms independent of binding to DNA.
The other oestrogen produced by the ovaries is oestrone, but this is synthesized mainly by the conversion of androstenedione in the peripheral tissues.
Progesterone
Progesterone is the principal hormone secreted by the corpus luteum and is responsible for ‘progestational’ effects, including induction of secretory activity in the endometrium in preparation for the implantation of a fertilized egg, inhibition of uterine contractions, increased viscosity of cervical mucus and glandular development of the breasts. Changes in progesterone levels also mediate the changes in basal body temperature during the ovulatory cycle. The basal body temperature increases by 0.3–0.5° C after ovulation, persists during the luteal phase and returns to normal after the onset of menses.
Other ovarian hormones
Inhibin is a glycoprotein consisting of two disulphide-linked subunits, alpha and beta. The beta -subunit can exist in two forms, and therefore there are two forms of inhibin—inhibin A and inhibin B. Inhibin B is secreted by the follicle and inhibits the release of FSH from the pituitary gland. Inhibin A levels are low in follicular phase and are increased in the luteal phase.
Activin is secreted by the follicles. It may enhance FSH secretion and may have local effects on ovarian steroid synthesis. Follistatin binds to and attenuates the action of activin.
Amenorrhoea
Amenorrhoea is the absence of menstrual periods in a woman during her reproductive years.
Amenorrhoea may be primary or secondary:
• Primary amenorrhoea is defined as the absence of menstrual periods by age 14 in a girl without breast development or by age 16 in a girl with breast development.
• Secondary amenorrhoe a is defined as the absence of menstrual periods for more than 3 months in a woman who has previously had an established menstrual cycle.
Epidemiology
The incidence of primary amenorrhoea is about 0.5–1.2%. The incidence of secondary amenorrhoea is about 5%.
Turner’s syndrome (see below) occurs in up to 1.5% of conceptions, 10% of spontaneous abortions and 1 in 2000–2500 live births.
Aetiology
Considerable overlap exists between causes of primary and secondary amenorrhoea. All causes of secondary amenorrhoea can also present as primary amenorrhoea.
Amenorrhoea may be due to defects at any level of the reproductive system: hypothalamus, pituitary, ovaries, uterus or vaginal outflow tract. Other causes of menstrual abnormalities include thyroid dysfunction and hyperandrogenism (Box 21.1).
Constitutional delay of puberty is an uncommon cause of delayed puberty and primary amenorrhoea in girls (see Chapter 29). It is difficult to distinguish clinically from congenital GnRH deficiency except that these girls eventually go on to have completely normal pubertal development at a later age.
Hypothalamic and pituitary disorders
Functional hypothalamic amenorrhoea (Box 21.1) is characterized by abnormal hypothalamic GnRH secretion, resulting in decreased gonadotrophin pulsations.
Pituitary/hypothalamic tumours and other infiltrative disorders (Box 21.1) may cause hypogonadotrophic hypogonadism and amenorrhoea (see Chapter 12).
Hyperprolactinaemia may be due to a prolactin-secreting pituitary adenoma or other tumours causing pituitary stalk compression. This interrupts the transport of dopamine to the anterior pituitary, which normally exerts an inhibitory effect on prolactin secretion. Hyperprolactinaemia can result in hypogonadotrophic hypogonadism by a direct inhibition of gonadotrophin release.
Congenital GnRH deficiency is a rare cause of primary amenorrhoea. Patients with congenital GnRH deficiency have apulsatile and prepubertal low serum gonadotrophin levels. Congenital GnRH deficiency associated with anosmia is called Kallmann’s syndrome. Kallmann’s syndrome may be due to sporadic or familial mutations of several genes (e.g. KAL1, FGFR1 [KAL2], PROK2) required for the migration of GnRH-secreting neurones into the hypothalamus. Other rare causes include Prader-Willi and Laurence-Moon-Biedel syndromes (see Chapter 29).
Congenital GnRH deficiency can be inherited as an autosomal dominant, autosomal recessive or X-linked condition. However, more than two-thirds of cases are sporadic.
Premature ovarian failure
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree