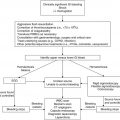
Clinical scenario
Recommendation
Level of evidencea
Risk stratification
Must be individualized at local institutions due to lack of evidence to support the needs of each individual treatment area
2C
Baseline laboratory recommendations
CBC/diff to assess level of neutropenia and monocytopenia in addition to other cytopenias; CMP for potential drug interactions; CVC culture or peripheral culture if no CVC; viral studies if symptomatic and appropriate season
1B
Utilization of DTP at FN presentation
Peripheral blood cultures in addition to CVC cultures can be done at the discretion of individual institutions but may not impact management
2C
Baseline imaging
Not recommended except CXR if with respiratory symptoms
1B
Antibiotic management at FN presentation in the clinically stable patient
Monotherapy with piperacillin/tazobactam, cefepime, or carbapenem; based on institutional preference, initial dual therapy with an antipseudomonal cephalosporin plus aminoglycoside (e.g., ceftazidime + tobramycin) can also be considered; aminoglycosides may be preferably administered once daily; initial antibiotic therapy should not include vancomycin unless there is concern for a Gram-positive infection due to such findings as sepsis, severe mucositis, skin infection, pneumonia or recent high-dose cytarabine administration
1A
Location of FN management
All patients should initially be managed as inpatients; if early discharge is considered, a multidisciplinary approach to determine appropriate risk stratification and outpatient therapy is required with IRB approval of such a prospective study to ensure safety and efficacy; patients identified as low risk may be discharged after 48 h per protocol if stable and without microbiologic-documented infection
1B
Management of low-risk patients
Empiric antibiotic therapy may be discontinued after 48 h if the patient is afebrile ≥24 h with signs of bone marrow recovery; patients may also be discharged after 72 h if afebrile ≥24 h even without signs of bone marrow recovery if close follow-up is ensured based on institutional preference
1B
Management of high-risk patients
Antibiotic therapy should be continued until resolution of FN episode
1A
FN ≥5 days
Empiric CT of the chest ± sinuses can be considered to rule out occult fungal infection; serial galactomannan can be considered
1C
FN ≥5 days in high-risk patients
Empiric antifungal therapy can be considered; appropriate empiric antifungal agent is unclear and must be chosen based on institutional preference; preemptive management may also be considered on a local basis as there is unclear evidence that prophylaxis prevents IFI
1C
1.2 History and Physical Examination
Critical assessment of the child with FN begins by obtaining a complete medical history and performing a thorough physical examination. This initial assessment will direct initial risk stratification and subsequent diagnostic evaluations (Table 1.2). It is important to inquire about the characteristics and height of the fever as temperature >39.0 °C has been noted as an independent risk factor for serious bacterial infection, and children (mainly inpatient) with a median fever of 39.4 °C have been reported to be at significantly increased risk of viridans streptococcal shock syndrome with underlying viridans strep bacteremia (Klaassen et al. 2000; Gassas et al. 2004). Fever may be the only sign of infection given the blunted inflammatory response associated with neutropenia. One must inquire about the presence of rigors or chills with central line flushing, recent therapy (potential for prolonged neutropenia), current medications (including antibiotic prophylaxis), and possible infectious exposures at home and school as well as recent travel (Orudjev and Lange 2002). Additional history should focus on community outbreaks of specific pathogens (i.e., respiratory viruses), prior history of fevers and documented infections, and pathogen colonization (e.g., methicillin-resistant Staphylococcus aureus [MRSA], vancomycin-resistant Enterococcus [VRE]). A comprehensive review of each organ system is important to determine possible etiologies of the fever. The physical exam should be thorough and not focus only on common sites of infection unique to the febrile neutropenic child (i.e., oral and perirectal mucosa, central venous access sites), but rather on all sites of common childhood infection such as the ears, throat, and skin (Auletta et al. 1999). Information obtained from the review of systems and physical findings will direct laboratory and ancillary evaluations and influence the choice of antimicrobial therapy.
Table 1.2
Diagnostic evaluation based upon organ system involvement and physical findings
Organ system | Physical findings | Diagnostic evaluation based upon physical finding(s) |
|---|---|---|
Head, ears, nose, and throat | Mucositis Thrush Oral lesions Pre-septal/orbital cellulitis Facial pain Rhinorrhea Otorrhea | HSV, VZV, Enterovirus/Parechovirus PCR/DFA/viral culture (PCR preferred) Biopsy unusual oral lesions (histology and microbiology) CT scan of the sinuses, orbits, temporal bones (obtain sample) Sinus drainage sample if able to perform and send for bacterial, fungal, and viral culture Nasopharyngeal secretions for respiratory viral culture/antigen panel and PCR |
Respiratory | Cough/respiratory distress Hypoxemia Chest radiograph infiltrates | CT chest Legionella urine antigen PCP evaluation: BAL, (1,3)-β-d-glucan, LDH Sputum culture if able to perform Aspergillus galactomannan, (1,3)-β-d-glucan Histoplasma urine and serum antigen |
Vascular access sites | Exit site/tunnel erythema or discharge | Blood culture from all ports (consider bacteria, fungus, mycobacteria) Gram stain and culture (bacteria, fungal) exit site discharge |
Gastrointestinal | Diarrhea Perirectal pain Abdominal pain | C. difficile PCR Viral stool culture/PCR (adenovirus, norovirus, etc.) SSYC and/or O&P if exposure by history Ultrasound or CT abdomen/pelvis Liver function test, amylase, lipase |
Skin | Rash Cellulitis | DFA/PCR/viral culture of vesicular lesion Dermatology evaluation and biopsy of cellulitis or undiagnosed rash/lesion and send for culture (bacterial, fungal, and atypical mycobacteria) |
Musculoskeletal | Arthritis Limp/point tenderness Lower back pain | Arthrocentesis: fluid for cell count and differential, cultures for bacteria, fungus, and mycobacteria CT/MRI extremity or site Urine culture (bacteria, fungal, consider adenovirus, BK, CMV PCR) |
Central nervous system | Change in mental status Headache | CT/MRI brain (consider MRA/V) Lumbar puncture: cell count, bacterial and fungal stain and culture, viral PCR (consider CMV, EBV, Enterovirus, HHV-6, HSV, VZV) |
1.3 Defining the Risk for Serious Infection
Risk stratification for serious infection incorporates the following variables: presenting clinical signs and symptoms, underlying cancer diagnosis and remission status, type of antitumor therapy received, and medical comorbidities (Orudjev and Lange 2002; Paganini et al. 2007; Freifeld et al. 2011). Sepsis signs and symptoms, presence of a central venous access device (CVAD), mucositis, infant acute lymphoblastic leukemia (ALL), acute myelogenous leukemia (AML), induction or intensification chemotherapy, and leukemia in relapse have all been shown to increase infection-related morbidity and mortality in pediatric patients and should be regarded as high-risk features at initial FN presentation (Orudjev and Lange 2002; Wicki et al. 2008; Badiei et al. 2011). Social factors including history of noncompliance and distance >1 h from clinical facility must be considered high-risk conditions (Orudjev and Lange 2002; Paganini et al. 2007). Paganini et al. (2007) showed that advanced disease stage, bacteremia, and associated comorbities including persistent bleeding, refractory hypoglycemia, hypotension, altered mental status, renal insufficiency and hepatic dysfunction were independent risk factors for mortality.
The Multinational Association for Supportive Care in Cancer (MASCC) risk index has been prospectively validated in adults allowing for potential outpatient management of low-risk patients (Klastersky et al. 2000; Uys et al. 2004). Multiple risk prediction models for pediatric oncology patients have been presented in the literature and are reviewed by Orudjev and Lange (2002), Härtel et al. (2007), Phillips et al. (2010), and Lehrnbecher et al. (2012). No one system has been found superior, none have undergone rigorous prospective validation to ensure patient safety across populations, and no prospectively validated stratification for high-risk patients has been identified (Härtel et al. 2007; te Poele et al. 2009; Phillips et al. 2010; Lehrnbecher et al. 2012). With the lack of significant randomized prospective data, Härtel et al. (2007) strongly recommend against outpatient management of FN in pediatric patients outside of clinical trials. In the recent pediatric FN expert consensus guidelines, Lehrnbecher et al. (2012) recommend the use of local population-based stratification systems with careful prospective and continuous evaluation to ensure safety and efficacy.
In particular risk stratification schemata, initial laboratory values such as platelet count <50 × 109/L and C-reactive protein (CRP) ≥90 mg/L have been noted to increase risk, while absolute monocytosis ≥0.1–0.155 × 109/L (depending on study) has tempered risk (Rackoff et al. 1996; Baorto et al. 2001; Santolaya et al. 2002; Ammann et al. 2010). Multiple laboratory assessments including CRP, procalcitonin, IL-6, and IL-8 have all been studied in pediatric and adult oncology patients to potentially define high-risk patients but none has been uniformly shown as an effective marker (Santolaya et al. 1994; Lehrnbecher et al. 1999; Uys et al. 2007; Semeraro et al. 2010; Phillips et al. 2012). These studies have been recently reviewed by Phillips et al. (2012).
1.4 Diagnostic Evaluation
1.4.1 Initial Laboratory Evaluation
The diagnostic evaluation for the pediatric patient with FN should be guided by clinical history and physical findings to optimize detecting an etiology for the fever. Initial work-up includes a complete blood cell count (CBC), a comprehensive metabolic panel, and blood cultures from each port of a central catheter. The CBC will demonstrate the degree of neutropenia and monocytopenia, and the metabolic panel will evaluate renal and hepatic function, which could be affected by previous chemotherapeutic agents and may influence antimicrobial selection and dosing. For example, if the patient’s creatinine is elevated, antimicrobials like aminoglycosides, vancomycin or amphotericin B should be used with caution and appropriate trough levels followed (Fisher et al. 2010; Lahoti et al. 2010).
Peripheral blood cultures should be obtained if cultures are not or are unable to be obtained from the patient’s central venous access device. Peripheral blood cultures in addition to central line cultures are recommended in infection guidelines but remains controversial as the yield for detection from peripheral cultures is <15 % and the utility of differential time to positivity (DTP) between central and peripheral cultures in treatment decision-making is unclear in FN (Gaur et al. 2003; Raad et al. 2004; Gaur et al. 2005; Mermel et al. 2009; Scheinemann et al. 2010; Lehrnbecher et al. 2012; Rodriguez et al. 2012; Carraro et al. 2013). To increase the blood culture yield, ≥1 mL of blood should be added to the culture bottles for children ≥1 month of age (Connell et al. 2007). Repeating blood cultures in patients who are persistently febrile has been shown useful in identifying bacteremia in a subset with initial negative cultures (Rosenblum et al. 2013). Most contemporary blood culture vials support the growth of Candida spp; however, other fungal species may not grow, so sending fungal blood cultures from all ports should be considered with persistent fever (i.e., ≥5 days) (Hennequin et al. 2002; Kosmin and Fekete 2008). If clinically suspected, acid-fast bacteria (AFB) cultures from the blood should also be sent to best isolate mycobacterial species.
Urinary tract infection (UTI) in the neutropenic patient may be subclinical given that patients may have minimal symptoms and pyuria may not be present in urinalysis (Klaassen et al. 2011). A recent study utilizing midstream urine samples reported UTI frequency of 8.6 % in 45 children with 58 episodes of FN, with all patients being asymptomatic and the majority having normal urinalyses, supporting the recommendation to obtain clean-catch bacterial urine cultures at the time of initial FN presentation (Lehrnbecher et al. 2012; Sandoval et al. 2012).
1.4.2 Radiographic Imaging
1.4.2.1 Chest Radiography (CXR)
A supine CXR may identify pleural effusions, allow for assessment of central line catheter position and detect pulmonary congestion. However CXR is less useful in identifying early pneumonia (Heussel 2011). CXR has not been found beneficial without the presence of respiratory symptoms and therefore should only be obtained in the symptomatic patient (Feusner et al. 1988; Roberts et al. 2012).
1.4.2.2 Computed Tomography (CT)
Institutional practice may recommend CT of the chest, abdomen and pelvis in neutropenic patients with ≥5 days of persistent fever although a recent pediatric study challenges this practice (Agrawal et al. 2011). Specifically, for 52 children with 68 episodes of FN for whom CT (sinuses, chest, abdomen and pelvis) was performed at day five of fever, minimal changes in clinical management occurred based upon results from imaging. The authors conclude that patients with prolonged FN (defined as >4 consecutive days of fever), in which occult fungal infections are being sought, should have CT imaging limited to the chest (Agrawal et al. 2011). Other pediatric studies have similarly found CT chest as well as sinuses to be most useful in prolonged FN (Archibald et al. 2001). CT of the sinuses can be performed in the context of pulmonary symptoms to rule out invasive fungal infections, as some patients with fungal sinusitis can present with minimal symptoms. Of note, patients with fungal sinusitis usually have positive findings on chest CT, and endoscopic biopsy of the sinuses has the highest yield for determining the microbiologic cause (Ho et al. 2011).
High-resolution chest CT with thin sections (1 cm slices) is the preferred imaging modality in patients with neutropenia and pulmonary symptoms, given its ability to characterize lesions and discriminate between infectious and noninfectious etiologies (Heussel 2011). In addition, CT imaging can be used for diagnostic purposes such as CT-guided biopsy of lung lesions for histologic and microbiologic assessment. Importantly, radiologic findings of common molds in children may differ from those in adults. For example, pulmonary nodules are common findings in pediatric patients with Aspergillus, while the halo and crescent signs occur less frequently than in adult patients (Thomas et al. 2003; Burgos et al. 2008). Therefore, consultation with pediatric radiologists is critical when interpreting these studies. Providing the radiologist with information about the patient’s clinical presentation, level of neutropenia, and current supportive therapy including specific antimicrobial and growth factor use increases the diagnostic utility of CT (Heussel 2011).
1.4.2.3 Magnetic Resonance Imaging (MRI)
MRI is advantageous over CT due to a lack of radiation exposure although at a much higher cost. MRI is also more affected by motion related to cardiorespiratory function increasing artifact incidence especially in the chest. Furthermore, given its increased imaging time, MRI usually requires sedation for pediatric patients, decreasing its convenience as a diagnostic tool. However, MRI remains the imaging of choice for assessing lesions in the liver, spleen, and central nervous system (CNS) (brain and spine) (Sahani and Kalva 2004; Luna et al. 2006).
1.4.2.4 Positron Emission Tomography (PET)
PET is now used frequently alongside CT for the diagnosis of cancer, but experience using PET/CT for FN is limited. A recent study from Australia compared diagnostic yields of PET/CT and conventional imaging in 20 adult patients with FN of >5 days duration (Guy et al. 2012). By using conventional imaging, the authors found 14 infections sites, 13 of which were also confirmed by PET/CT; in addition, PET/CT identified 9 more sites of infection (8 of which were subsequently confirmed as true infection), suggesting that PET/CT may be useful in the study of prolonged FN. However, more data are needed to support the use of this modality as first-line imaging in pediatrics given its inherent expense and associated radiation exposure (Xu et al. 2010; Haroon et al. 2012).
1.4.3 Biomarkers for Invasive Fungal Infection (IFI)
Early identification of infection in FN, especially IFI, critically affects patient survival. Biomarkers for IFI have proven useful in the adult population as adjuncts to clinical findings and imaging. Limited pediatric data suggest these biomarkers may also be used in children with similar sensitivities and specificities as defined in adults.
1.4.3.1 Aspergillus Galactomannan (GMN)
GMN is a cell-wall component of growing hyphae and can be detected by assay from the serum, urine and bronchoalveolar (BAL) fluid (Klont et al. 2004; Pfeiffer et al. 2006). The GMN assay has been shown effective in adult populations with hematologic malignancies and limited data in children suggest utility in pediatric oncology patients as well (Sulahian et al. 2001; Pfeiffer et al. 2006; Steinbach et al. 2007; Castagnola et al. 2010). A recent observational, prospective, multicenter study found the specificity of urine and serum GMN assays were 80 % and 95 %, respectively. This study found that the false-positive rate was lower than previously described (Fisher et al. 2012).
The GMN assay must be interpreted with caution, particularly in the context of a single positive value, and should be utilized in conjunction with corroborative clinical and radiologic findings. Serial repetition of the assay can be used as a surveillance marker either for potential IFI or disease response to antifungal therapy (Groll et al. 2014). The GMN assay may have false-positive results in patients receiving beta-lactam antimicrobials (especially piperacillin/tazobactam), although recent studies suggest the false-positive rate may not be as high as previously published (Zandijk et al. 2008; Metan et al. 2010; Mikulska et al. 2012).
1.4.3.2 (1,3)-β-d-glucan (BDG)
Serum BDG, an important cell-wall component of most fungi including Pneumocystis jiroveci (former carinii), has been measured in adult patients with IFI and hematologic malignancies (Marty and Koo 2009). Like GMN, studies using serum BDG in the pediatric population are limited. In fact, few studies performed to date even establish a normal value for children, which may be higher than the 60 pg/mL cutoff used in adult patients (Smith et al. 2007; Mularoni et al. 2010). In immunocompromised adult patients, serum BDG levels exceeding 500 pg/mL have been used to diagnose P. jiroveci (Del Bono et al. 2009; Koo et al. 2009; te Poele et al. 2009). One pediatric study found serum BDG potentially useful for P. jiroveci diagnosis in three patients with hematologic malignancies, one with BAL fluid confirmation (Gonzalez et al. 2011). The sensitivity of BAL for diagnosing P. jiroveci pneumonia is lower in non-HIV patients and in patients receiving aerosolized pentamidine. Therefore, serum BDG may be a useful adjunctive, noninvasive diagnostic tool (Levine et al. 1992; Azoulay and Schlemmer 2006; Jiancheng et al. 2009). More pediatric studies are needed to define serum BDG as a reliable indicator of IFI such as P. jiroveci.
Serum BDG does not detect Cryptococcus or Zygomycetes spp. (e.g., Mucor, Rhizopus, Absidia), which do not produce BDG. False positives also occur in patients receiving antimicrobial agents such as piperacillin/tazobactam in addition to hemodialysis with cellulose membranes, intravenous albumin and immunoglobulin (Marty and Koo 2009; Karageorgopoulos et al. 2011). Finally, the test may serve as a prognostic marker for invasive candidiasis when serial levels are measured, but data supporting this indication are limited (Ginocchio et al. 2012; Glotzbecker et al. 2012; Jaijakul et al. 2012).
1.4.3.3 Polymerase Chain Reaction (PCR)
Molecular methodology such as PCR testing may improve the detection of IFI as well as bacterial or viral organisms in children with FN (Santolaya et al. 2011; Kourkoumpetis et al. 2012). However, lack of standardization has limited its current use and more data are required to make firm recommendations.
1.4.4 Viral Studies
Viral infections may cause prolonged fevers in neutropenic patients. Seasonal viral infections such as influenza A/B and respiratory syncytial virus (RSV) in the winter and enterovirus in the summer must be considered (Lindblom et al. 2010; Ozdemir et al. 2011). In patients with mucositis, herpes simplex virus (HSV) should be considered. Viral PCR (whole blood and plasma, cerebrospinal fluid, stool) is commercially available and enables faster identification of viral etiologies at higher sensitivity and specificity compared to viral culture. In addition, direct fluorescent antibody (DFA) testing of cutaneous lesions may expedite diagnosis of herpetic skin infections (i.e., HSV, varicella).
1.4.5 Invasive Procedures: Bronchoalveolar Lavage (BAL) and Tissue Biopsy
Tissue is necessary for diagnosis when physical examination or radiographic imaging is concerning for abscess formation or lesions of the skin, sinuses, or organ parenchyma but without corroborative microbiologic confirmation. Microbiologic evaluations should include proper sample collection for assessing AFB and other bacterial and fungal pathogens. For IFI such as chronic disseminated candidiasis or invasive Aspergillus spp., hepatic or splenic biopsy may be required (Masood and Sallah 2005).
In children with pulmonary lesions, BAL should be attempted first as it has a low complication rate and may yield an etiologic agent (Pattishall et al. 1988; Jain et al. 2004; Efrati et al. 2007). CT-guided lung biopsy or wedge resection may be necessary if BAL sampling is nondiagnostic in the patient with persistent fever and pulmonary nodules (Wingard et al. 2012). In addition to routine cultures, BAL samples should be analyzed by GMN assay (Wingard et al. 2012).
In patients with neutropenia, skin lesions may be a manifestation of localized or systemic infection (Mays et al. 2006). Ecthyma gangrenosum, a black eschar with surrounding erythema originally attributed to Pseudomonas spp., can be caused by many other bacterial as well as fungal and viral pathogens (Moyer et al. 1977; Reich et al. 2004; Son et al. 2009). Many invasive systemic fungal infections with high potential for dissemination like Zygomycetes spp., Aspergillus spp. and Candida spp. may present as nonspecific lesions that require prompt evaluation (Mays et al. 2006). Contaminated medical equipment such as adhesive tape has been associated with nosocomial outbreaks of Zygomycetes spp., so it is imperative to consider such infections in skin lesions found under dressings near tape (Everett et al. 1979; Lalayanni et al. 2012). Prompt punch biopsy and consideration for consultation with a dermatology specialist is advisable.
1.5 Empiric Management of Febrile Neutropenia (FN)
Management of FN in pediatric oncology is often based on institutional and consensus guidelines. To inform decision-making we review the appropriate literature regarding specific topics including the use of monotherapy versus combination antibiotic therapy, use of vancomycin, empiric utilization of antifungals, emergence of resistant pathogens, duration and location of therapy, and criteria for central venous catheter removal. Pediatric FN guidelines from one institution are provided as an example of how these concepts can be practically implemented.
1.5.1 Adult FN Guidelines for Empiric Therapy: Do They Apply to Children?
Infectious Diseases Society of America (IDSA) guidelines for empiric antimicrobial therapy for FN patients serve as the foundation for institutional protocols treating pediatric cancer patients (Freifeld et al. 2011). Notably, the International Pediatric Fever and Neutropenia Guideline Panel has also published guidelines for FN in pediatric oncology patients (Lehrnbecher et al. 2012). Recommendations from such practice guidelines are mostly based upon level III evidence (expert opinion) versus level I and II evidence (results from randomized clinical trials) (Lee and Vielemeyer 2011). Furthermore, no formal guidelines for FN have been published by the American Society of Pediatric Hematology/Oncology (ASPHO) or the Pediatric Infectious Diseases Society (PIDS) despite notable disparity among adult and pediatric cancer patients, including differences in underlying malignant diseases and associated therapies, immunity and susceptibility to pathogens, and antimicrobial pharmacodynamics (PD) and pharmacokinetics (PK) (Sung et al. 2011; Watt et al. 2011). Defining such PD/PK differences are critical, particularly in the cancer patient as cancer therapy can affect antimicrobial efficacy, promoting pathogen resistance (Theuretzbacher 2012). Whether such differences significantly impact infection-related morbidity and mortality in pediatric cancer patients remains largely unstudied.
The International Antimicrobial Therapy Cooperative Group (IATCG) published the largest series comparing FN episodes in adult (n = 2,321) and pediatric (n = 759) patients receiving standardized disease assessment and empiric therapy and noted significant differences in patient demographics and outcomes by age: (1) malignant diagnoses associated with FN episodes differed across patient age with ALL being most frequent in children and AML most frequent in adults; (2) adult patients more frequently received antibacterial and antifungal prophylaxes; (3) children tended to have lower ANCs at presentation but shorter durations of granulocytopenia; (4) children had less defined sites of infection and more fever of unknown origin; (5) children and adults had similar rates of Gram-positive and Gram-negative bacteremia, but children had more streptococcal bacteremia; and (6) children had lower infection-related mortality and overall mortality (3 % vs. 10 %) than adult FN patients (Hann et al. 1997). Together, these data suggest that children and adults with FN are indeed distinct both in presentation and outcome.
Additional studies are needed to investigate further these suggestive data. However, such clinical investigation is practically limited by the large numbers of patients required for accurate statistical analysis as well as by the inherent expense associated with large randomized clinical trials (RCTs) (Mullen 2012). Likewise, reproducible and accurate biomarkers of response to infection and clinical end points are needed to ensure sound clinical trials addressing antimicrobial efficacy and safety (Powers 2012). Given their limitations in study design and expense, RCTs comparing FN episodes and response to therapy among adult and pediatric cancer patients will likely not be performed. Yet, extensive pediatric literature incorporating antimicrobial agents used in adult FN demonstrates that empiric antibacterial and antifungal therapies are comparable in their efficacy. For these reasons, extrapolation of adult FN guidelines to the pediatric population is unavoidable.
1.5.2 Choice of Empiric Antimicrobial Therapy
As discussed, determination of initial risk stratification can help guide utilization of appropriate antimicrobial agents and specifically the route (oral vs. intravenous), setting (inpatient vs. outpatient), and duration of use. In general, antimicrobial choices for empiric pediatric FN are comparable in their efficacy in either high-risk or low-risk scenarios as serious medical complications remain low with use of contemporary treatments (Baorto et al. 2001; Luthi et al. 2012; Manji et al. 2012b, c). Therefore, institutional guidelines for empiric antimicrobial therapy should consider the following: (1) published experience with using the antimicrobial agent in the context of FN; (2) physician experience with using the proposed antimicrobial agent; (3) pathogen epidemiology and resistance patterns to the antimicrobial agent inherent to the institution and its surrounding community; and (4) the antimicrobial agent’s toxicity profile, cost and availability. In essence, choice of empiric antimicrobial therapy should integrate the patient’s clinical history and presentation with institutional experience of local microbial patterns and specific antimicrobial therapies.
1.5.2.1 Monotherapy Versus Combination Therapy
Pizzo et al. (1986) first showed that cephalosporin monotherapy is as effective as combination therapy in adult oncology patients. Since that time, institutional guidelines are slowly adopting the recommendation for monotherapy that is supported in both the IDSA as well as the recent pediatric guidelines (Freifeld et al. 2011; Lehrnbecher et al. 2012). In a Cochrane review of 71 published trials, monotherapy with broad-spectrum, antipseudomonal beta-lactams was found to be non-inferior to combination therapy with a trend toward improved survival and a significantly decreased risk of adverse events, specifically fungal infection and nephrotoxicity secondary to aminoglycosides as part of combination therapy (Paul et al. 2013). The observations of Paul et al. (2013) have been corroborated by a previous systematic meta-analysis by Furno et al. (2002). Specifically for pediatric oncology patients, Manji et al. (2012b) conducted a meta-analysis and found no significant difference between antipseudomonal penicillins and antipseudomonal cephalosporins either as monotherapy or when combined with an aminoglycoside. The authors therefore recommend choosing a regimen based on cost, availability and local factors such as institutional resistance patterns. Whether the increased utilization of a more broad-spectrum agent as monotherapy over a more narrow-spectrum antipseudomonal cephalosporin plus an aminoglycoside will lead to increased resistance for these agents is unknown.
In an additional Cochrane review of anti-Gram-positive antibiotics (often vancomycin) in FN, Paul et al. (2005) showed that the addition of such therapy does not improve outcomes without a documented Gram-positive infection. Use of vancomycin as initial empiric FN therapy is not recommended in consensus guidelines as it does not significantly affect survival or length of stay (Freifeld et al. 2011; Lehrnbecher et al. 2012). Furthermore, imprudent use of vancomycin has been associated with emergence of resistant pathogens (e.g., VRE) and nephrotoxicity. Clinical indications for empiric vancomycin include skin/soft-tissue and catheter-related infection, hemodynamic instability, severe mucositis, and pneumonia. In these situations, targeted vancomycin trough levels and renal function surveillance are recommended (Rybak et al. 2009). If susceptible bacteria are not recovered or if concern for Gram-positive infection abates, vancomycin should be discontinued within 72 h (Lehrnbecher et al. 2012).
Consensus guidelines recommend combination therapy be reserved for specific clinical indications including patient instability, concern for resistant pathogens (e.g., extended spectrum β-lactamase [ESBL]-producing Serratia, Pseudomonas, Acinetobacter, Citrobacter, Enterobacter and Klebsiella spp.), and need for synergism to treat specific pathogens (e.g., Enterococcus, Mycobacterium spp., MRSA) or infections (e.g., endocarditis, cryptococcal meningitis) (Freifeld et al. 2011; Lehrnbecher et al. 2012). Of note, if combination therapy is required, meta-analyses in pediatrics recommend utilization of once rather than multiple daily doses of aminoglycosides due to trends toward improved efficacy and decreased nephrotoxicity (Sung et al. 2003; Contopoulos-Ioannidis et al. 2004).
1.5.2.2 Which Monotherapy to Choose
A Cochrane review of antipseudomonal beta-lactams for initial management of FN compared studies with ceftazidime, cefepime, piperacillin/tazobactam, meropenem and imipenem (Paul et al. 2010). Cefepime monotherapy was shown to have a significantly higher all-cause mortality which had been previously reported (Yahav et al. 2007). The Cochrane meta-analysis reported a nonsignificant higher rate of bacterial superinfection with cefepime which has been a reported concern due to its limited anaerobic profile and poor coverage for skin infections (Yahav et al. 2007; Paul et al. 2010; Kalil 2011). A follow-up meta-analysis did not find a statistically significant increased mortality with cefepime and current pediatric consensus guidelines consider cefepime a reasonable choice for monotherapy (Kim et al. 2010; Lehrnbecher et al. 2012). Ceftazidime is not a good first-line choice for monotherapy due to reduced Gram-positive coverage as well as induction of β-lactamase production leading to subsequent emergence of resistant pathogens and inferior clinical outcomes in pediatric patients (Mebis et al. 1998; Ariffin et al. 2000; Greenberg et al. 2005). Paul et al. (2010) additionally noted that carbapenem monotherapy had similar all-cause mortality as other monotherapy regimens but was associated with higher rates of antibiotic- and Clostridium difficile-associated diarrhea. Pediatric meta-analyses have shown similar effectiveness between antipseudomonal cephalosporins, antipseudomonal penicillins and carbapenems as monotherapy but without the noted increase in Clostridium difficile-associated diarrhea (Manji et al. 2012b, c).
1.5.2.3 Alterations in Initial Empiric FN Antibiotic Management
Once empiric therapy has been initiated, alterations in FN antibiotic management may be required to optimize treatment; these changes occur at the discretion of the practitioner or institution without significant evidence basis. For instance, Lehrnbecher et al. (2012) recommend discontinuing combination therapy (if initiated at presentation) after 24–72 h in the stable patient without microbiologic evidence to continue both agents. Similarly, patients who are stable but with persistent fever should not have their initial regimen escalated. Those who become unstable should have additional coverage for potential resistant Gram-negative, Gram-positive and anaerobic causes initiated with consideration for fungal and viral etiologies.
1.5.2.4 Outpatient Management of FN
Although there remains a lack of one uniform pediatric oncology risk stratification system, many institutions have begun to utilize outpatient management of low-risk FN which can include outpatient oral or parenteral therapy as either initial management or as step-down to outpatient treatment after initial inpatient management; such options and the evidence surrounding them are reviewed by Chisholm and Dommett (2006). This differs from previous studies in which low-risk patients were discharged early (ANC <0.5 × 109/L) but only after defervescence (Mullen and Buchanan 1990; Aquino et al. 1997; Wacker et al. 1997). Meta-analyses of efficacy and safety from adult and pediatric studies found no significant difference in treatment failure in the inpatient versus outpatient setting and no significant difference in the efficacy of outpatient oral versus parenteral therapy in low-risk FN (Teuffel et al. 2011b). Studies were extremely heterogenous in terms of choice of antibiotics (both oral and parenteral) as well as timing of step-down making generalizations difficult. A recent Cochrane review of randomized controlled trials comparing oral versus intravenous antibiotic therapy for FN found no significant difference in treatment failure or mortality (Vidal et al. 2013).
The utilization of outpatient oral therapy specifically for pediatric patients either at FN presentation or as step-down after initial inpatient management was most recently reviewed by Manji et al. (2012a). Sixteen prospective trials were reviewed in the meta-analysis with no significant difference between oral and parenteral regimens and no outpatient infection-related mortality. None of the trials were randomized controlled trials specifically comparing inpatient versus outpatient management and outcomes of low-risk FN (Manji et al. 2012a). The types of oral agents utilized in pediatric studies are quite heterogenous and include amoxicillin/clavulanate, cefixime, fluoroquinolones (ciprofloxacin, gatifloxacin) and combination therapy (ciprofloxacin plus amoxicillin) (Mullen et al. 1999; Aquino et al. 2000; Paganini et al. 2000, 2001; Shenep et al. 2001; Park et al. 2003; Petrilli et al. 2007; Dommett et al. 2009; Brack et al. 2012). Ciprofloxacin plus amoxicillin/clavulanate is recommended as the oral regimen of choice in adult patients (Freifeld et al. 2011). Brack et al. (2012) recently reported on an RCT comparing continued inpatient treatment versus oral outpatient management and found non-inferiority for efficacy but lack of power to prove non-inferiority for safety.
Many centers are continuing to initially admit all pediatric oncology FN patients with the potential for early discharge with or without continued antibiotic support depending on the clinical context (Gibson et al. 2013). The United Kingdom recommends such a management strategy after 48 inpatient hours in patients >1 year of age, without medical or social comorbidities, not receiving extremely intensive therapy, appearing clinically well without a source of infection, with some marrow recovery (i.e., ANC >0.1 × 109/L), and with fever improvement (but not full defervescence necessary), for outpatient oral antibiotics to complete a 5-day course (Gibson et al. 2013). A recent survey of Canadian pediatric oncology centers found heterogenous treatment strategies from full inpatient care, to step-down care, to full outpatient care exemplifying the perceived lack of sufficient data to uniformly modify practice (Boragina et al. 2007). Sung et al. (2004) reported in a survey of parents that only 53 % supported initial FN outpatient management (as compared to 71 % of practitioners) due to perceived increased fear and anxiety balanced with increased comfort, while early discharge and outpatient intravenous management are reportedly associated with improved health-related quality of life (Cheng et al. 2011). Finally, Teuffel et al. (2011a) calculated that the most cost-effective model is one in which low-risk FN patients are treated entirely at home but through a parenteral rather than oral route. The risk of nosocomial infection (NI) with inpatient management must also be considered and was reported to be 5.2 NI per 100 admissions by one group (Simon et al. 2000). The recent pediatric FN expert consensus guidelines allow for consideration of initial or step-down outpatient management for the low-risk patient in the appropriate setting although as a weak recommendation (Lehrnbecher et al. 2012).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree