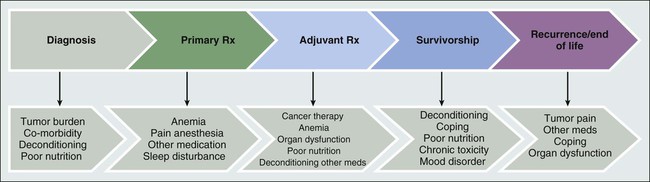
Joanna M. Brell and Lee W. Jones • Fatigue affects most patients with cancer, regardless of the stage of disease or the type of anticancer therapy used. • Cancer-related fatigue (CFR) affects an estimated 80% to 90% of outpatients with cancer who receive either chemotherapy or radiation therapy. • CRF can be persistent in cancer survivors. • Determining the exact incidence requires standardized methods to define, diagnose, and measure CRF. • Specific pathophysiological mechanisms are unknown at this time but undoubtedly are multifactorial. • CRF may be caused by the malignancy and/or anticancer therapy. • CRF is exacerbated by other conditions, some of which are controllable, such as anemia, hypothyroidism, and depression. • CRF may affect compliance to and tolerance of anticancer therapies. • CRF impairs function, self-care, ability to work, social relationships, and quality of life. • No standardized measurement tools exist except for International Classification of Diseases–10 criteria, which have not been widely adopted. • Using National Comprehensive Cancer Network guidelines, patients report levels of fatigue using a numeric rating scale of 0 to 10. This numeric rating scale is currently used as a screening tool. • No Food and Drug Administration–approved interventions are currently available. • All treatable factors of fatigue should be addressed. • Patients reporting fatigue should be enrolled in clinical studies relating to CRF whenever possible. • The best evidence to date for improved outcomes related to CRF involve cardiopulmonary fitness through exercise, but further research in this area is needed. • Stimulants may help severely fatigued patients near the end of life. Cancer-related fatigue (CRF) is experienced by most patients with cancer at any point from the time of diagnosis, during treatment, through survivorship, or at disease progression. The National Comprehensive Cancer Network (NCCN) guidelines consensus definition of CRF is a “distressing persistent, subjective sense of physical, emotional and/or cognitive tiredness or exhaustion related to cancer or cancer treatment that is not proportional to recent activity and interferes with usual functioning.”1 Fatigue with these characteristics does not appear to occur in the general population. In a large analysis of almost 35,000 patients, fatigue was measured with use of the Functional Assessment of Chronic Illness Therapy (FACIT) questionnaire and comparisons were made between anemic patients with cancer, nonanemic patients with cancer, and a cohort from the general population (with or without medical conditions) (N = 1010). The patients with cancer, regardless of their hemoglobin level, experienced significant fatigue (P > .001) compared with persons without cancer.2 CRF can negatively affect functional status, resulting in limitations in ambulation and exercise, oral intake, socialization with supportive friends and families, ability to maintain a job, and quality of life. Of critical importance, fatigue may prevent full doses of anticancer therapy from being delivered, which could potentially affect survival or tumor control. The exact incidence of CRF depends on when in the cancer trajectory it occurs and by what methods patient-reported experience is measured. It does not appear that any time exists on the cancer continuum when patients are not challenged by fatigue (see Fig. 45-1), and CRF occurs with all types of cancers and in all stages. Most likely CRF is underreported, because both patients and health care providers expect fatigue as an inevitable result of a malignancy diagnosis. Discussions between patients and physicians regarding fatigue may be limited, given that few efficacious interventions exist to improve fatigue. In a review of 27 publications in which CRF was the primary outcome, the incidence ranged from 4% to 91%.3 This wide range probably is the result of significant variation in the patient populations studied, including type of malignancy, stage of disease, receipt of cancer therapy, the type of treatment modality received, and patient gender. In addition, these studies used various survey tools to record patients’ specific description of their fatigue. Currently, 14 validated scales exist to measure CRF, and each has a different construct for evaluating fatigue.4 In all, it has been estimated that 80% to 90% of outpatients with cancer who received either chemotherapy or radiation therapy report the presence of various levels of fatigue.5 Additionally, severe fatigue has been recorded in 75% of hospitalized patients on a palliative care unit.3 One population studied extensively has been breast cancer survivors. In a study of more than 750 breast cancer survivors, almost 20% of patients who were free of disease for 5 to 10 years after completing therapy had significant residual fatigue that impaired their ability to function.6 With new anticancer therapeutic agents causing or associated with fatigue, longer treatment periods, and an increasing number of survivors, CRF is currently and will continue to be a substantial clinical issue. The causes and contributing factors of fatigue are undoubtedly multifactorial, and thus its etiology is not well delineated. Understanding the causative factors is important for finding effective clinical therapies or preventative strategies for CRF. Patients with cancer experience multiple comorbid conditions, in addition to the malignancy itself, that can cause or exacerbate fatigue. This combination of factors may prevent or delay detection of the ultimate contributing disorders. Patients may acquire these medical conditions after receiving therapy or they may have these conditions at the time of presentation with cancer; the term “cancer-related” can encompass all of these factors, because the exact time of onset in the cancer trajectory may be unknown. Defining CRF is quite challenging because many patients with cancer present with fatigue before primary adjuvant therapy is begun. Regardless of the source, clinically recognizing separate causes of fatigue at presentation will help uncover treatable situations, such as anemia, hypothyroidism, depression, concomitant medications, and infection. Remediating potentially reversible conditions is the initial approach to CRF according to NCCN guidelines.1 The literature regarding possible CRF mechanisms contains a few small clinical trials and little preclinical work. The published data suggest multiple nonspecific interactions between cancer, the effects from cancer therapy, and the patients’ responses to both. Unfortunately, the consequences of these interactions have not been convincingly correlated to patient report of fatigue. As well, findings of putative mechanism-directed therapies have not been successful to date. One possible mechanism currently being studied, however, involves the inflammatory response to cancer and its therapy, especially cytokine activation. Clinical studies have revealed increases in interleukin-1 (IL-1) receptor antagonist and C-reactive protein in fatigued patients with cancer who are undergoing radiation therapy.7 CFR has also been correlated with tumor necrosis factor (sTNF-RII), immunoregulatory CD4 lymphocytes, and white blood cell subsets.8 Proinflammatory proteins have been noted in breast cancer survivors with no evidence of disease who have experienced fatigue 3 to 5 years after cancer therapy.9 The reasons why inflammatory responses are still maintained after treatment and resolution of the cancer have not been fully explained. In a study of a group of breast cancer survivors with or without fatigue, at least one cytokine polymorphism at IL-1β–511 (P = .007) was associated with reported fatigue.10 Regardless of the quality or quantity of the pathophysiology involved, patients consistently note multiple sensations that often occur not in isolation but in clusters of complaints. Given the interwoven nature of the facets of CRF, causative perturbations may exert a systemic effect on multiple organs and tissues to obtain the phenotype of fatigue. Clinically, this cluster appears similar to inflammatory-induced symptoms referred to as “sickness behavior” and may encompass depression, sleep disturbances, and cognitive dysfunction.11 A sickness behavior animal model induced by lipopolysaccharide injection has been associated with behavioral changes (e.g., listlessness, decreased oral intake, and sleep disturbances) and increased levels of circulating proinflammatory cytokines.12 Although this preclinical model has been widely used for CRF studies, a lack of valid preclinical models of fatigue remains. The discovery of new animal models of fatigue continues to be a challenging yet key aspect of CRF research.13 Because CFR is subjective, the patients’ description of fatigue (at the bedside) should guide the generation of hypotheses (at the bench) to begin to uncover the etiology or etiologies of CRF, even though some questions surrounding CRF mechanisms do not easily lend themselves to preclinical assessment. Modeling human situations such as lack of motivation to perform, comorbid conditions, and concomitant medications cannot yet be recapitulated. Valid preclinical models can help to accelerate an improved mechanistic understanding of fatigue. This understanding will allow for more precise interventions to be brought into clinical testing. Several grants and research projects are currently exploring inflammation as the primary driver of CRF. Patients with cancer are often older and commonly present with a diverse range of age-related changes, similar to the general adult population, that limit cardiorespiratory fitness. However, these normal aging consequences are dramatically compounded by the consequences of anticancer therapies, tumor burden in advanced disease, comorbid conditions such as antecedent cardiac disease and pulmonary insufficiency, and decreased activity/deconditioning.14 These situations simultaneously affect the O2 cascade. A growing body of evidence shows that patients with cancer have significant impairments in cardiorespiratory fitness.15 The data have been collected by methods using objective determination of cardiopulmonary function,16 thus providing reproducible, standardized information. International guidelines defining the proper conduct of cardiorespiratory fitness testing have also been developed.19–19 An incremental cardiopulmonary exercise test with gas exchange measurement to assess peak oxygen consumption (VO2peak) constitutes the gold standard for assessment of cardiorespiratory fitness. Although routine use of cardiopulmonary exercise testing is not feasible, it is a highly informative research tool to measure physiological processes that may deepen the understanding of CRF. One study reported that the mean VO2peak in 346 presurgical patients with non–small cell lung cancer was 25% to 44% below that for age- and sex-matched normative data for sedentary persons.20 As well, the mean VO2peak was 27% below that of age-matched sedentary healthy persons compared with patients with stages I to IV breast cancer, with the lowest mean VO2peak seen in women with metastatic cancer. Additionally, it was determined that a 40-year-old woman with breast cancer has a cardiopulmonary capacity similar to that of a healthy sedentary 70-year-old woman.21 It is not clear if these observed marked reductions in cardiorespiratory fitness are due to limitations in pulmonary, cardiovascular, or peripheral tissues. However, the impairment is probably multifactorial with no one single organ component of O2 transport/utilization yet implicated as being solely responsible.14 It may be that a combination of exercise plus pharmacologic and psychosocial interventions could be tested for their role in initiating and maintaining CFR. If damage to some aspect of the cardiorespiratory system by cancer or its treatment were contributory, then it follows that either measure of the individual organ components and/or the total functional capacity of these organs (cardiorespiratory fitness) should be correlated with patient-reported fatigue. Jones et al.22 recently reported that in patients with newly diagnosed malignant glioma, prior to therapy, decreased peak VO2peak was correlated with patient-reported fatigue (measured by the FACIT fatigue scale). Also, measures of body composition such as lean body mass, as well as total muscle cross-sectional area by magnetic resonance imaging, were inversely correlated with reported fatigue. In a subsequent study, functional capacity, as measured by a 6-minute walk test, was inversely correlated with fatigue in 171 patients with recurrent malignant glioma.23 Similar findings have been reported in other cancer populations with different measures of cardiorespiratory fitness and functional capacity, including patients with advanced lung cancer24 and early-stage breast cancer.25 These studies support the correlation between CRF and diminished cardiorespiratory activity and strengthen the hypothesis that increasing physical function during or immediately after therapy (primarily through physical exercise) may have a significant effect on reducing CRF. Fatigue is an experienced sensation. According to NCCN guidance, patients are asked to assess their current level of CRF on a 0 to 10 numeric rating scale (NRS) that is portioned as follows: 0, no fatigue; 1-3, mild fatigue; 4-7, moderate fatigue; and 8-10, severe fatigue.1 This NRS is a screening tool that has also been utilized as an eligibility criteria measurement for fatigue in patients prior to clinical trial enrollment. Based on the results of this screening tool, NCCN has published guidelines for evaluating patients with fatigue that includes reassessment for any changes in fatigue.1
Fatigue
Definition
Incidence
Etiology
Evaluation
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Oncohema Key
Fastest Oncology & Hematology Insight Engine