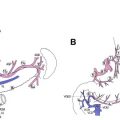
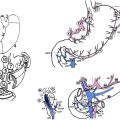
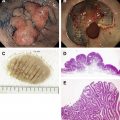
This article focuses on the diagnosis and management of familial gastric cancer, particularly hereditary diffuse gastric cancer (HDGC). First, existing consensus guidelines are discussed and then the pathology and genetics of HDGC are reviewed. Second, patient management is covered, including surveillance gastroscopy, prophylactic total gastrectomy, and management of the risk of breast cancer.
An army marches on its stomach. —Napoleon Bonaparte 1769–1821
The Bonapartes are probably the most notorious reported gastric cancer family. However, recent analysis of Napoleon’s family history and pathology records challenges the assumption of inherited predisposition. Two hundred years ago, the practice of medicine largely rested on the arts of taking an accurate history and sharp clinical observation. Today, despite the explosion in molecular and genetic knowledge, this is still the case and a meticulous family history remains a cornerstone of diagnosis in familial cancer.
Although hereditary diffuse gastric cancer (HDGC) is relatively rare, sporadic, gastric cancer, it is the fourth most common cancer worldwide. In 1998, germline mutations in the E-cadherin gene ( CDH1 ) were first described in three gastric cancer families from New Zealand. In the year after this discovery, different CDH1 mutations were identified in other gastric cancer families from around the world, culminating in the definition of the first and, to date, only inherited cancer syndrome dominated by gastric cancer, HDGC. Despite extensive mutation searching in other candidate genes, no further mutations have been found in families in which gastric cancer predominates.
Parry Guilford and colleagues identified the mutant CDH1 gene, in part, because the pedigree of the index family (a large Maori family known as Family A) was well documented, permitting an accurate genetic linkage analysis. Forty years before this, in 1964, Dr Ted Jones, a young resident doctor at Tauranga Hospital had published the tragic story of multiple deaths in very young family members from a diffuse type of stomach cancer. In 1965, Pekka Lauren, of the eponymous Lauren Classification, published his seminal work on the different morphologic types of gastric cancer.
Lauren described the distinction between stomach cancers that form glands (eg, cancers of the colonic intestine), which he called the “intestinal type,” and the “diffuse type” in which discohesive malignant cells invade in single files, sheets, or nests of cells without forming a discrete mass. Epithelial-cadherin is a cell-to-cell adhesion molecule that is a central component of the adherens junction. The difference in E-cadherin expression between gastric cancer histotypes was first demonstrated immunohistochemically by Shimoyama and Hirohashi in 1991. Their study showed that in diffuse gastric cancer (DGC) E-cadherin expression was occasionally absent, but more often reduced or abnormal. Subsequently, Becker and colleagues supported this by demonstrating that CDH1 mutations are restricted to sporadic gastric cancers with diffuse morphology.
Later work demonstrated that inactivating E-cadherin mutations are exclusively observed in the diffuse component of mixed gastric carcinomas. In a commentary on this paper and other literature, Chan and Wong highlighted that loss of E-cadherin provides a “plausible explanation” for the divergent morphologic phenotype in lobular versus ductal breast cancer and DGC versus intestinal gastric cancer (IGC). In Newfoundland, Canada, a germline CDH1 mutation was detected in another very large family; however, they were originally identified as a breast cancer family.
This article focuses on the diagnosis and management of familial gastric cancer, particularly HDGC. First, existing consensus guidelines are discussed and then the pathology and genetics of HDGC are reviewed. Second, patient management is covered, including surveillance gastroscopy, prophylactic total gastrectomy, and management of the risk of breast cancer.
Consensus guidelines
In 1999, the first guidelines on diagnosis and management of familial gastric cancer were published by the International Gastric Cancer Linkage Consortium (IGCLC). In New Zealand, because of the particularly young age at which HDGC patients have died, guidelines were needed on the youngest age at which genetic testing, surveillance gastroscopy and prophylactic gastrectomy are recommended. In response, at the 2004 meeting of The New Zealand Familial Gastric Cancer Group (scientists, clinicians, and allied health professionals ) consensus guidelines were established based on collective clinical experience managing these families and the literature. At that time, there were 45 HDGC families reported worldwide, 10 from New Zealand.
In the initial 5 years after CDH1 mutations were described, several key papers were published on genetic counseling, the cumulative risk of gastric and breast carcinoma, prophylactic gastrectomy, early gastric pathology, and surveillance chromogastroscopy.
In 2008, at the seventh workshop of the IGCLC, updated consensus guidelines were generated. Management algorithms have been formulated highlighting the salient management decisions. Now, there are well over 100 HDGC families reported in the literature. The information from the published pedigrees of the first 87 families reported (up to 2008) is summarized in Table 1 including mutation details, number of gastric and breast cancers, known histotypes, age at diagnosis, and cancers at other sites.
| Family Identification | Ethnicity: Country | CDH1 Mutation | Type | Exon Intron | Mutation Effect | GC (DGC) | Age of GC Cases | Mean Age | Breast (LBC) | Age of Breast Ca | Other Cancers | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C7 | ns: North America | 3G>C | Non | 1 | No protein | 1 (1) | 73 | NA | 1 (1) | 73 | — | Suriano 2005 |
| 203 | European | 45insT | ins | 1 | Truncation | 4 (2) | 30–67 | 44 | 1 (1) | — | 3xCo (1xSRC) | Oliveira 2002 |
| — | White | 49–2A>C | ss | In 1 | — | 4 (3) | 42, 54, 57, 68 | — | 1 | 55 | Cr, S, Pr, Bl, Pa, T | More 2007 |
| — | White: UK | 49–2A→G | ss | in 1 | truncation | 6 (4) | 34–69 | 49 | nil | — | Lu, Co | Richards 1999 |
| — | ns: Irish | As above | “ | “ | “ | 2 (2) | ns | — | ns | — | — | Moran 2005 |
| C220 | White | 53delC | del | 2 | truncation | 2 (2) | 28–48 | 38 | nil | — | Lu, Cx | Humar 2002 |
| — | White: UK | 59G→A | non | 2 | truncation | 3 (3) | 27–50 | 38 | nil | — | — | Richards 1999 |
| 4201 | White: USA | 70G→T | non | 2 | truncation | 4 (3) | 37–46 | 42 | 3 | 39, 39, 46 | Leu x2 (45, 66y) | Guilford 1999 |
| — | Japanese | 185G→T | mis | 3 | missense | 7 ? | 46–72 | 59 | nil | — | — | Shinmura 1999 |
| — | White: USA | 187C>T | non | 3 | truncation | 3 (2) | 33–85 | 62 | 1 | — | 2x Co, Pr, 2xEn | Gayther 1998 |
| F6 | North American | As above | “ | “ | “ | 3 (1) | 24–52 | ? | 3 (2) | 39, 39, 56 | LBC 39×2, 1 mixed ca | Suriano 2005 |
| DST | Maori: NZ | 190C→T | non | 3 | truncation | 2 (2) | 22, 28 + others | ? | — | — | — | Guilford 1999 |
| — | White: French | 283C→T | non | 3 | truncation | 4 (3) | 34, 42, 43, 47 | ? | 2 | 64, +ns | + ‘digestive ca’ at 25y | Dussaulax 2001 |
| F1 | Unknown | As above | “ | “ | “ | 7 (2) | 39–76 | — | — | — | ns | Kaurah 2007 |
| F2 | White | 353C>G | mis | 3 | missense | 2 (1) | 55, 63 | — | nil | — | — | More 2007 |
| — | White: German | 372delC | del | 3 | truncation | 4 (2) | 15, 37, 38, 58 | 37 | 1 (1) | 49 | — | Keller 1999 |
| F10 | Italian | 382delC | del | 3 | — | 7 (1) | 32, 40, 42, 45, 50, 55, 56 | 46 | 2 | — | — | Brooks-Wilson 2004 |
| — | White | 531+2T>A | ss | In4 | truncation | 5 (3) | 36, 53, 48, 22, 23 | — | 1 | 43 | 4xcleft lip or palate | Frebourg 2006 |
| RS | White: France | 586G→T | non | 5 | truncation | 4 (4) | 31–55 | 45 | nil | — | — | Guilford 1999 |
| F1 | Northern European | 687(+1)G>A | ss | Int 5 | — | 4 (1) | 36, 40, 48, 50 | 43 | 1 | — | — | Brooks-Wilson 2004 |
| — | Caucasian | 715G>A | Mis,ss | 6 | missense | 2 (2) | 30, 29 | — | nil | — | 1 mutation +ve alive at 79 | More 2007 |
| F2 | Filipino | As above | “ | “ | “ | 4 (1) | 29–33 | — | nil | — | — | Karurah 2007 |
| G2 | Korean | 731A→G | mis | 6 | missense | 7 ? | ?38–63 | ? | nil | — | — | Yoon 1999 |
| 215 | Finnish | 808T>G | mis | 6 | missense | 1 | Late 70s | — | nil | — | HPC: 4xPr in 70s | Ikonen 2001 |
| 195 | Pakistani | 832G→A | ss | 6 | truncation | 10 (6) | 23–70 | 37 | 1 | — | — | Oliviera 2002 |
| F26 | Northern European | 892G→A | mis | 7 | missense | 3 (2) | 32, 33, 36 | 34 | — | — | — | Brooks-Wilson 2004 |
| 23 | Swedish | 1003C→T | Non | 7 | truncation | 3 (2) | 22, 44, 45 | — | 1 (0) | 51 ductal | Pr x1 86y | Jonsson 2002 |
| F1 | North American | As above | “ | “ | “ | 8 (2) | 25–67 | — | nil | — | ns | Suriano 2005 |
| F3 | North American | As above | “ | “ | “ | 5 (1) | 35–55 | — | nil | — | ns | Suriano 2005 |
| A | Maori: NZ | 1008G→T | ss | 7 | truncation | 28 (9) | 14–74 | 33 | 2 (1) | Bi lob 43, 49 | Co | Guilford 1998 |
| 185 | European | 1018A>G | mis | 8 | missense | 2 | 35–47 | 39 | — | — | Ovary | Oliviera 2002 |
| F25 | Northern European | 1064insT | ins | 8 | — | 3 (2) | 34, 50, 58 | 47 | nil | — | — | Brooks-Wilson 2004 |
| — | Hispanic | 1107delC | del | 8 | Truncation | 3 (2) | 27, 48, ? | — | Nil | — | — | More 2007 |
| — | Italian | 1118C>T | mis | 8 | Missense | 3 (3) | 70, 72, 79 | — | nil | — | Co x 1 | Roviello 2007 |
| F41 | Spanish | 1134del8ins5 | Del-in | 8 | — | 4 (3) | 27, 30, 32 + b | — | nil | — | — | Brooks-Wil 2004 |
| SF1 | Italian: Brazil | 1137G>A | ss | 8 | ns | 3 (2) | 18, 28, 37 | — | nil | — | Cleft palate, aplasia cutis, partial acrania | Frebourg 2006 |
| F5 | White | As above | “ | “ | “ | 1 (1) b | ns b | — | Unknown | — | More 2007 | |
| F6 | White: Swiss | As above | “ | “ | “ | 1 (1) | 29 | — | 1 | 69 | Saliv gland 26y, Co, 2x bilateral lung | More 2007 |
| F3 a | unknown | As above | “ | “ | “ | 7 (1) | 26-44 | — | 1 | — | — | Kaurah 2007 |
| F4 a | Sweden or Norway | As above | ‘ | “ | “ | 3 (2) | 37–48 | — | nil | — | — | Kaurah 2007 |
| chg72 | African American | 1137+1G→A | ss | Int 8 | truncation | 5 (4) | 25–58 | 42 | — | — | — | Guilford 1999 |
| F9 | Northern European | 1212delC | del | 9 | — | 5 (4) | 17, 32, 46, 47, 61 | 41 | 4 | — | — | Brooks-Wilson 2004 |
| F7 | Northern European | 1226T→C | mis | 9 | missense | 1 (1) | 51 | 51 | — | — | SRC colon | Brooks-Wilson 2004 |
| — | Japanese | 1243A>C | mis | 9 | missense | 5 (2) | 60–63 | 62 | — | — | — | Wang 2003 |
| — | White | 1397-92delTC | Del | 10 | truncation | 2 (2) | 34, 61 | — | 1 | 78 | — | More 2007 |
| F5 | English | As above | “ | “ | — | 4 (2) | 31–82 | — | 1 | 62 | — | Kaurah 2007 |
| G1001 | Korean | 1460T→C | mis | 10 | missense | 2 ? | ? 42–49 | — | nil | — | — | Yoon 1999 |
| PQ036 | European | 1472-73insA | ins | 10 | truncation | 3 (2) | 32–40 | 35 | nil | — | — | Oliviera 2002 |
| F18 | Northern European | 1476delAG | Del | 10 | — | 2 (1) | 32–40 | 36 | nil | — | — | Brooks-Wilson 2004 |
| — | Chinese | 1507C>T | non | 10 | Truncation | ns (1) | — | — | 1 (1) | — | Proband DGC and LBC | Jiang 2004 |
| C230 | Arab | 1565+1G→T | ss | Int 10 | truncation | 3 (2) | 49–56 | 52 | nil | — | — | Humar 2002 |
| 1000 | White: USA | 1588insC | ins | 11 | truncation | 3 (3) | 40–63 | 50 | 1 | — | — | Guilford 1999 |
| — | Spanish | 1610delC | del | 11 | truncation | 3 (3) | 50, 58, 71 | — | 1 (1) | <50y | Pr, Co | Rodriguez 2006 |
| — | European or German | 1619insG | ins | 11 | — | 1 (1) | 29 | 29 | 1 | 49, bilat | Ab, Lu | Keller 2004 |
| F6 | Irish | 1682insA | ins | 11 | — | 3 (1) | 38–52 | — | 1 (1) | 38y + DGC | Note 38y DGC & LBC | Kaurah 2007 |
| C210 | African American | 1710delT | del | 11 | truncation | 2 (2) | 19, 29 | 24 | 1 | — | Pr | Humar 2002 |
| — | White: USA | 1711 insG | Ins | 11 | truncation | 9 ? | 30–68 | 45 | nil | — | — | Gayther 1998 |
| F11 | Northern European | 1711 + 5G>A | ss | In11 | — | 3 (3) | 44–48 | 45 | 5 (1) | — | — | Brooks-Wilson 2004 |
| HPc31 | Sweden | 1774G>A | mis | 12 | unknown | 2 | 69, 85 | — | 2 | 46, 54 (1-ve) | Pr x4 (mean age 69) 1xCo | Jonsson 2002 |
| F2 | Northern European | 1779insC | Ins | 12 | — | 3 (3) | 33–42 | 37 | — | — | — | Brooks-Wilson 2004 |
| — | White: Canada | 1792C→T | Non | 12 | truncation | 6 (3) | 23–43 | 31 | — | — | — | Gayther 1998 |
| C200 | Maori: NZ | As above | “ | “ | “ | 3 (1) ? | 30–41 | 35 | — | — | Pan | Humor 2002 |
| F5 | North America | As above | “ | “ | “ | 4 (1) | 30–54 | — | 2 | — | — | Suriano 2005 |
| SF2 a | Portuguese | 1901C→T | mis | 12 | missense | 1 (1) | 30 | – | nil | — | — | Suriano2003 |
| 32SF a | Portuguese | As above | “ | “ | “ | 2 | 23, 26 | — | nil | — | — | Oliviera 2004 |
| F7 | English: no linkage | As above | “ | “ | “ | 2 (1) | 34, 45 | — | 1 | 75 | — | Kaurah 2007 |
| F8 | Maori | As above | “ | “ | “ | 2 (2) | 25, 30s | — | nil | — | — | More 2007 |
| F8 | Spanish | 1913G>A | Non | 12 | ns | 10 (1) | 21–59 | — | nil | — | — | Kaurah 2007 |
| 16, SF4 | Northern European | 2061delTG | del | 13 | — | 2 (2) | 24, 47 | 36 | nil | — | — | Brooks 04+Kaurah |
| SF9 | English or Scottish | As above | “ | “ | — | 3 (1) | 37–80 | — | 2 | 65? | — | Kaurah 2007 |
| C | Maori: NZ | 2095C→T | non | 13 | truncation | ns | ns | ns | — | — | — | Guilford 1998 |
| F9 | Chinese | As above | “ | “ | “ | 3 (3) | 24, 39, 55 | — | — | — | — | More 2007 |
| — | North American | 2161C>G | ss | 13 | — | 4 (1) | 21–75 | — | nil | — | — | Suriano 2005 |
| F10 | Unavailable | 2164 + 5G>A | ss | In13 | — | 3 (1) | 38–44 | — | nil | — | — | Kaurah 2007 |
| F13sf5 | Northern European | 2195G→A | mis | 14 | missense | 2 (2) | 36–70 | 53 | 4 (2) | 44–86 | Note br ca both families | Brooks 04+Kaurah |
| F11 | English: no linkage | As above | “ | “ | “ | 3 (2) | 32–65 | — | 4 (1) | 40–77 | Note br ca both families | Kaurah 2007 |
| F12 | Colombian | 2245C>T | mis | 14 | Missense | 3 (2) | 36–49 | — | nil | — | — | Kaurah 2007 |
| F2 | North American | 2276delG | del | 14 | — | 10 (2) | 35–87 | — | 2 (1) | — | — | Suriano 2005 |
| 240 | White | 2295 + 5G→A | ss | Int 14 | truncation | 4 (2) | 44–52 | 47 | nil | — | — | Humar 2002 |
| F4 | Northern European | 2310delC | Del | 15 | — | 8 (≥1) | 42–79 b | 61 b | 1 | — | — | Brooks-Wilson 2004 |
| HPC | Swedish | 2329G>A | mis | 15 | n.s | 1 | 74 | — | nil | — | Pr x 3 (mean 68y) | Jonsson 2002 |
| F13 | English | 2343A>T | mis | 15 | — | 2 (2) | 51, 63 | — | 1 | ns | — | Kaurah 2007 |
| B | Maori: NZ | 2381insC | ins | 15 | truncation | 7 (2) | 30 (16–35) b | 30 b | — | — | — | Guilford 1998 |
| — | German | 2396C→G | mis | 15 | — | 3 (1m) | 41–52 b | 47 b | — | — | — | Keller 2004 |
| SF6, 7 F14,15 | Newfoundland founder mutation | 2398delC | del | 15 | ns | 29 (7) | 25–80 | — | 16 (4) | 41-68 | Cox8, Leux3, head or neck2, My, Lu, Br, Es, NHL, | Kaurah 2007 |
| — | Japanese | 2494G→A | mis | 16 | missense | 7 (1) | ns | ns | — | — | — | Yabuta 2002 |
| — | White | 2440-6C>G | ss | In 15 | — | 2 (2) | 36, ns | — | nil | — | Lu | More 2007 |
| — | Summary | 87 families 68 mutations | — | — | 30% miss 70% trun | — | 5 with case <20y, 9 with case <20–24 | — | 75 (18) | Youngest case 38y | — | — |
| Solitary Cases | ||||||||||||
| — | European: Canada | 41delT | del | 1 | — | 1 (1) | 30 | — | — | — | — | Bacani 2006 |
| C5 | North American | 1063 del 6 | del | 8 | Truncation | 1 (1) | 27 | — | — | — | — | Suriano 2005 |
| C6 | North American | 1285 C>T | mis | 9 | missense | 1 (1) | 27 | — | — | — | — | Suriano 2005 |
| MFW | Not specified | 1487del7 | del | 10 | truncation | 1 (1) | 31 | — | — | — | Father: co at 60y | Guilford 1999 |
| g71 | African American | 1849G→A | mis | 12 | missense | 1 | 64 | — | — | — | — | Ascano 2001 |
| 293 | African American | 1849G→A | mis | 12 | missense | 1 | 43 | — | — | — | Not related to 294 | Suriano 2003 |
| 294 | African American | 1849G→A | mis | 12 | missense | 1 | 43 | — | — | — | Not related to 293 | Suriano 2003 |
| Breast Cancer Family | ||||||||||||
| LBC | USA | 517insA | ins | 4 | truncation | nil | — | — | 2 (2) | 28, 42 | Melanomax2, kidney | Masciari 2007 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree