Extremity Soft-Tissue Sarcomas
Michael T. Selch
Introduction
Soft-tissue sarcomas arise from extraskeletal connective tissue. These neoplasms are unusual despite the fact that soft tissues comprise over 40% of adult body weight. It is estimated that 10,600 new soft-tissue sarcomas occurred in 2009, accounting for 1% of all nonskin malignancies (1). These tumors can affect any portion of the body, including the rare visceral sarcomas. Excluding visceral tumors, soft-tissue sarcomas arise in the following locations: 40% to 50% lower extremity; 30% trunk (retroperitoneum, mediastinum, chest wall, abdominal wall, and breast); 10% to 15% upper extremity; 10% head and neck (2,3). Approximately 75% of all extremity soft-tissue lesions arise proximal to the elbow or knee joints.
The incidence of soft-tissue sarcomas demonstrates a bimodal age distribution. The median age varies from 52 to 62 years but soft-tissue sarcomas also represent 7% of all pediatric cancers (4,5). Adult sarcomas predominantly affect the limbs while pediatric tumors arise primarily in the pelvis or head and neck. There is a slight male predilection for soft-tissue sarcomas but no apparent racial difference. Soft-tissue sarcomas have been reported in association with chronic lymphedema, various genetic anomalies (Li-Fraumeni, von Recklinghausen’s), environmental exposures (phenoxyacetic acid herbicides), and ionizing radiation but their etiology remains obscure.
Soft-tissue sarcomas were traditionally considered resistant to radiation therapy and their management largely depended upon surgical ablation. Local control following primary resection of an extremity soft-tissue sarcoma is related to the extent of surgery and the resulting margin obtained. Enneking and associates have defined four types of surgical margins for sarcoma surgery and the procedures required to achieve these margins (6). Intralesional excision is followed by local relapse in over 90% of cases (7,8). Marginal and wide excisions are accompanied by local recurrence in ∼70% and 40%, respectively (9,10). Surgical therapy is successful only when a radical margin is obtained. This specific margin is achieved when the tumor and surrounding normal tissues are removed by dissection through at least one uninvolved tissue plane in both the longitudinal and transversal dimensions. Satisfying these surgical constraints has resulted in local relapse rates as low as 2% to 18% (11,12). Achieving a radical margin, however, historically required amputation in nearly one-half of patients. Those patients radically resected, but not amputated, also suffered significant cosmetic and functional tissue loss affecting quality of life.
Current management of extremity soft-tissue sarcomas typically involves function-preserving surgery plus adjunctive radiation therapy (limb salvage therapy). Following a relatively conservative wide excision of an extremity sarcoma, radiotherapy will be required to eliminate likely microscopic residual tumor cells. It is known from the pioneering studies of Gilbert Fletcher on epithelial cancers that minimal amounts of neoplastic clonogens can be more effectively controlled by radiotherapy than large bulky deposits (13). Cells comprising soft-tissue sarcomas, furthermore, are no longer considered inherently “radioresistant.” In an in vitro model, the surviving fraction of fibrosarcoma cells after a 2-Gy exposure (SF2Gy) is equivalent to adenocarcinomas of the breast (14). Since the initial experience with limb-sparing therapy, randomized and nonrandomized trials have demonstrated local control rates for sarcomas at least equivalent to those reported for radical surgery (5,15,16,17,18,19,20,21,22). A randomized trial performed by the National Cancer Institute demonstrated adjunctive radiotherapy is necessary in the setting of limb-sparing resection (23). The investigators randomized 91 patients with high-grade extremity sarcomas and 50 with low-grade tumors to limb-sparing surgery with or without postoperative external beam radiotherapy. After a median 10-year follow-up, the local control rate among patients with high-grade tumors was 100%, following postoperative radiotherapy compared to 78% after limb-sparing surgery alone (p = 0.003). For those with low-grade neoplasms, the respective local control rates were 95% and 70% (p = 0.016). Although the patients entered on these salvage protocols are not necessarily comparable to those previously undergoing radical surgeries, these data imply that limb-sparing approaches offer a viable alternative to local management of extremity sarcomas in selected situations. The best candidates for this procedure are those in whom a gross total excision can be performed with preservation of a reasonably functional extremity. The purpose of this chapter is to describe the radiation therapy techniques that ensure successful multimodal management of extremity soft-tissue sarcomas in adults.
Natural History
An extremity soft-tissue sarcoma typically presents as a painless mass situated deep within limb musculature. Symptoms occasionally encountered include pain, paresthesia, and edema. The rarity of these tumors and the resulting lack of clinical suspicion result in a diagnostic delay varying from 4 to 28 months (2).
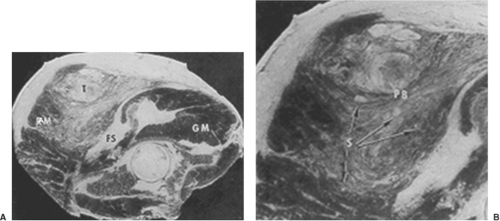
Soft-tissue sarcomas demonstrate slow centrifugal expansion. This radial growth produces a peripheral pushing border composed of atrophic normal cells and edematous reactive tissue. The resulting compressed tissue mimics tumor encapsulation on gross inspection. It has become abundantly clear that the compressive zone is not an effective barrier to microscopic tumor extension (Fig. 34.1). The compressive zone is more properly a “pseudocapsule” commonly penetrated by sarcoma cells (6).
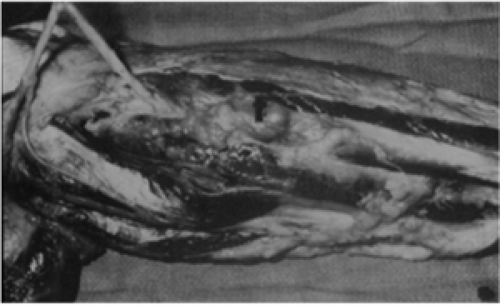
The normal extremity is composed of various muscle groups. Muscles with similar function are separated from other muscles by fascial barriers called major intermuscular septae. Although not strictly correct, these muscle groups and their accompanying neurovascular structures can be thought of as forming individual muscle compartments within an extremity (6). Muscles lying within a specific compartment are separated from each other by minor intermuscular septae. The growth of extremity soft-tissue sarcomas generally respects certain fascial barriers such as major intermuscular septae, interosseous membranes (tibia-fibula; ulna-radius), periosteum, perineurium and adventitia. Soft-tissue sarcomas arising within an extremity extend longitudinally, rather than radially, along the major fascial planes within the compartment of origin. As a consequence, undisturbed soft-tissue sarcomas generally do not extend transversally into the neighboring compartments until quite late in their course. Extremity sarcomas can, however, extensively permeate minor septae and infiltrate intracompartmental muscle bundles for a considerable distance from the primary lesion (Fig. 34.2). As a consequence of the natural growth pattern of sarcomas, removal of the tumor plus pseudocapsule very likely leaves microscopic residual neoplasm within the remaining intracompartmental structures for an uncertain distance from the resection bed. Extremity soft-tissue sarcomas arising in an extracompartmental location, such as the popliteal fossa, inguinal area, or axilla, encounter fewer
fascial restrictions and their pattern of spread is even less predictable. The subcutaneous tissue is also considered an extracompartmental location. Soft-tissue sarcomas arising in this area, however, usually display a less aggressive course than those lesions arising deep within the extremity.
fascial restrictions and their pattern of spread is even less predictable. The subcutaneous tissue is also considered an extracompartmental location. Soft-tissue sarcomas arising in this area, however, usually display a less aggressive course than those lesions arising deep within the extremity.
Soft-tissue sarcomas of the extremity rarely involve regional lymphatics. Mazeron reviewed 2,500 literature cases and reported a 4% incidence of lymph node involvement over the entire course of the disease (24). Elective lymph node dissection reveals unsuspected sarcomatous adenopathy in 6% to 13% of patients (2,25,26). Lymphatic involvement appears higher for certain histotypes (rhabdomyosarcoma, epithelioid sarcoma, and angiosarcoma), although this observation may be more related to other histopathologic features than a real biologic difference. Lymph nodes are rarely an isolated site of sarcoma relapse following local tumor management.
Pathology and Staging
Soft-tissue sarcomas are traditionally classified according to their presumed cell of origin. This histogenic classification scheme, proposed by Stout, defines the common following subtypes of tumors: fibrosarcoma, malignant fibrous histiocytoma, liposarcoma, leiomyosarcoma, rhabdomyosarcoma, angiosarcoma, lymphangiosarcoma, synovial cell sarcoma, and neurofibrosarcoma. All are of mesenchymal derivation except the latter which is of ectodermal origin. Tumors of uncertain histogenesis include alveolar soft part sarcoma, epithelioid sarcoma, clear cell sarcoma, and extraskeletal Ewing’s sarcoma. The histogenic grouping is not an accurate predictor of the metastatic potential of soft-tissue sarcomas. The most consistent predictor of biologic behavior is sarcoma grade (28,29,30). Virtually, all histotypes of sarcoma display a spectrum of histologic grade. Occasionally, this grade spectrum can be noted within an individual tumor. Certain histotypes present more commonly as a particular grade. For instance, the liposarcoma is generally low grade while the angiosarcoma is usually high grade.
Grading schemes in use today are based on analysis of mitotic rate, necrosis, cellular pleomorphism, differentiation, and cellularity. Sarcomas are divided into low-, intermediate-, and high-grade tiers. Grade is the prime determinant of tumor stage according to the American Joint Commission on Cancer (Tables 34.1 and 34.2) (31). Grade is also a predictor of lymphatic involvement and pulmonary metastases at diagnosis (24). The radiation oncologist must also remember other determinants of local relapse: tumor size, tumor location, surgical margin status, and skin involvement by a deep tumor (32,33). These factors may not have prognostic impact independent of tumor grade but will influence treatment planning in the individual patient.
Recent attention has focused on molecular biology determinants of prognosis. The presence of a t(x – 18) translocation is a significant predictor of metastasis-free survival for patients with synovial cell sarcoma (34). The impact of this translocation on local control, however, has not been analyzed. Overexpression of human telomerase is significantly more frequent in a group of locally recurrent soft-tissue sarcomas compared to those that are controlled (35). The influence of telomerase expression on local control has not been investigated prospectively. Finally, overexpression of aberrant p53 is a significant predictor of local recurrence for osseous chondrosarcoma (36). The influence of mutated p53 on the local relapse rate of soft-tissue sarcomas has not been studied. It is hoped that advances in molecular biology of soft-tissue sarcomas may lead to delineation of a subgroup of patients who are at extremely low risk of local recurrence and may, therefore, avoid adjunctive radiotherapy in the setting of limb-sparing surgery.
Table 34.1 American Joint Committee on Cancer Staging Classification for Soft-Tissue Sarcomas | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Table 34.2 American Joint Committee on Cancer Staging Classification for Soft-Tissue Sarcomas | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||
Although the prognostic significance of tumor histogenesis remains unreliable, the radiation oncologist should be cognizant of the peculiar growth patterns of specific extremity soft-tissue sarcomas. The angiosarcoma and lymphangiosarcoma typically arise in more than one compartment, suggesting a neoplastic process affecting an entire embryonic limb bud (Fig. 34.3). These cases are rarely amenable to nonamputative therapy or limited volume irradiation. The epithelioid sarcoma commonly arises on the hand or foot and is notorious for discontinuous spread (“skip metastases”), implying the requirement for wide-field irradiation regardless of tumor size (Fig. 34.4).
Diagnosis and Evaluation
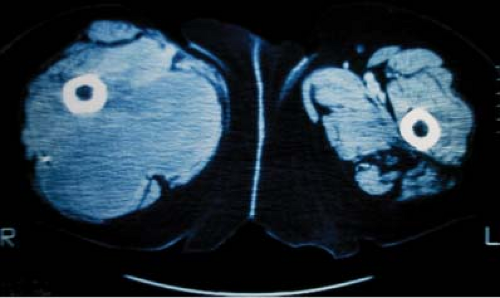
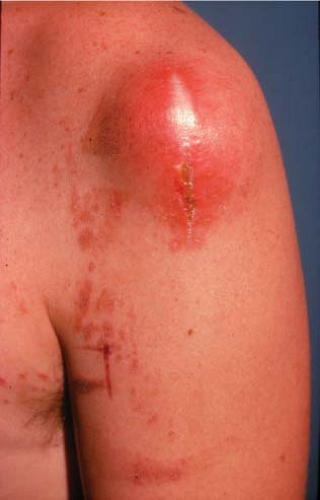
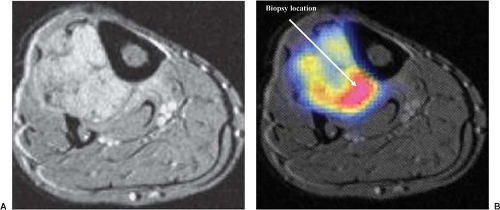
Definitive diagnosis of an extremity soft-tissue sarcoma requires tissue obtained by biopsy. Proper radiotherapy planning depends upon the knowledge of the type of biopsy procedure utilized. Excisional biopsy (“shelling out”) is appropriate only for lesions <4 cm in diameter and located superficially. Such lesions are generally benign or low-grade sarcomas with an indolent course such as the subcutaneous malignant fibrous histiocytoma. Rarely, an aggressive soft-tissue lesion such as the angiosarcoma will present superficially in the limb. Aggressive superficial sarcomas are more common in the trunk or head and neck. The majority of extremity soft-tissue sarcomas arise deep in the limb and are >5 cm in diameter. Incisional biopsy is the most appropriate procedure in this setting. Attempts at excisional biopsy of large, deep tumors are to be condemned (4). Excisional biopsy of this type of lesion may be associated with extensive postoperative eccymoses (Fig. 34.5). Areas of discoloration must be considered contaminated by sarcoma cells, thus vastly increasing the definitive surgical bed and radiotherapy field. Inappropriate biopsy also renders some limbs unsalvageable (37). Correct placement of the biopsy is also critical to the radiotherapy planning. The incision must be placed in the long axis of the limb and directly over the tumor (Fig. 34.6). A transversal approach, or one requiring extensive tunneling, may violate compartments not initially seeded with tumor. The transversal approach necessarily enlarges both the definitive surgical procedure and any radiotherapy field (Fig. 34.7). The entire biopsy site must be included in any patient undergoing preoperative radiotherapy and must be removed in toto during definitive resection. Image-guided core needle biopsy may supplant open procedures, provided the pathologist is skilled at the interpretation of small volume specimens (Fig. 34.8) (38). Positron emission tomography (PET) fused with computed tomography (CT) or magnetic resonance imaging (MRI) may assist in directing biopsy to the region of a heterogeneous tumor likely to harbor the highest grade elements (Fig. 34.9).
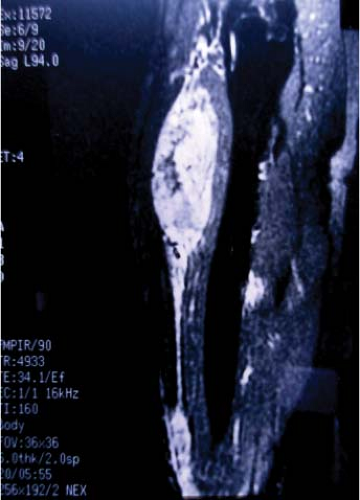 Figure 34.4. Coronal contrast-enhanced MRI demonstrating multifocal epithelioid sarcoma of the thigh (arrows). |
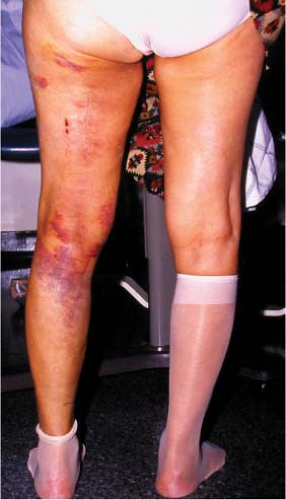 Figure 34.5. Extensive subcutaneous ecchymoses following excisional biopsy of a deep 8-cm sarcoma of the posterior thigh. |
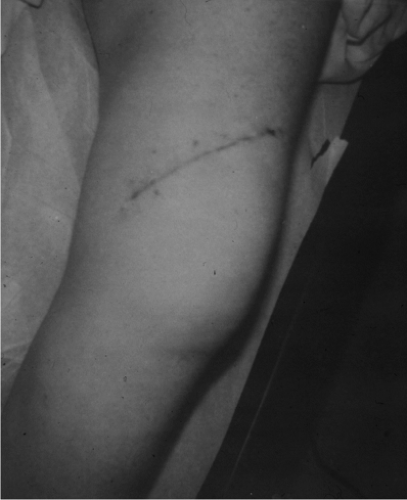 Figure 34.7. Oblique incision used for biopsy of a medial thigh mass. The incision extends into the adjacent anterior compartment. |
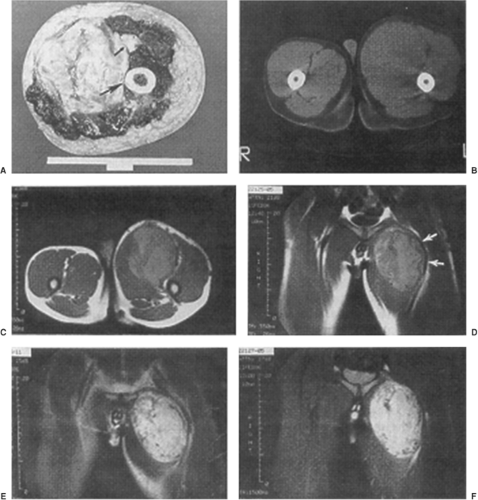
Radiotherapy planning requires accurate assessment of tumor extent within the extremity. Plane radiographs, isotope bone scans, and angiography provide minimal clues to the topographic extent of extremity soft-tissue sarcomas (Fig. 34.10). Bone scans may reveal uptake near a sarcoma. Since skeletal invasion by a soft-tissue sarcoma is extremely unusual, this activity is misleading (5). Increased uptake is due to periosteal inflammatory response to an adjacent sarcoma and is not an absolute indication for amputation (Figs. 34.11 and 34.12). Pretreatment evaluation of extremity sarcomas demands modern imaging. Magnetic resonance (MR) scanning is superior to CT (39,40,41,42). MR yields better spatial resolution of tumor extent than CT and displays this in the three standard planes of reference (Fig. 34.13). MR yields superior definition of normal tissue planes, neurovascular bundles, and normal tissue–tumor interfaces. Prior biopsy procedures may exacerbate peritumoral edema resulting in an overestimation of tumor extent (43). Gadolinium administration may increase MR specificity in this situation. CT remains superior to MR only for detection of true bone invasion. It is a general rule that a sarcoma with both osseous and soft-tissue components represents a primary bone tumor with soft-tissue extension rather than a primary soft-tissue tumor with bone invasion. Neither MR nor CT accurately predicts tumor histogenesis or grade. These modern imaging studies must be available to the radiation oncologist in the simulator room and planning computer to expedite
coverage of tissues at risk for tumor invasion with simultaneous exclusion of uninvolved normal tissue. PET is useful for the diagnosis of sarcoma and evaluation of tumor response to neoadjuvant therapies. The value of PET for radiotherapeutic treatment planning has not yet been established. Canadian investigators compared FDG-PET target volumes with T1-contrast enhanced MRI volumes in a series of 17 extremity soft-tissue sarcomas. For the purposes of target delineation on the PET study, threshold values of 2- to 10-times background and the 40% of maximum
uptake threshold were used. The authors found poor correlation between the PET-defined and MRI-defined volumes regardless of the threshold employed (44). Mean ratio of the GTVPET/GTVMRI ranged from 1.16 using a threshold value of 2 to 0.22 using a threshold of 10.
coverage of tissues at risk for tumor invasion with simultaneous exclusion of uninvolved normal tissue. PET is useful for the diagnosis of sarcoma and evaluation of tumor response to neoadjuvant therapies. The value of PET for radiotherapeutic treatment planning has not yet been established. Canadian investigators compared FDG-PET target volumes with T1-contrast enhanced MRI volumes in a series of 17 extremity soft-tissue sarcomas. For the purposes of target delineation on the PET study, threshold values of 2- to 10-times background and the 40% of maximum
uptake threshold were used. The authors found poor correlation between the PET-defined and MRI-defined volumes regardless of the threshold employed (44). Mean ratio of the GTVPET/GTVMRI ranged from 1.16 using a threshold value of 2 to 0.22 using a threshold of 10.
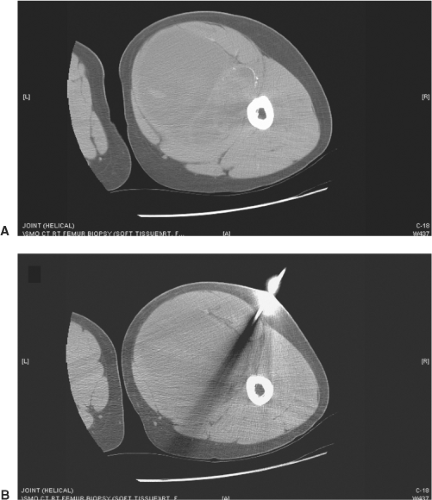 Figure 34.8. A: Axial CT demonstrating heterogeneous soft-tissue mass in the right thigh. B: Axial CT demonstrating core needle biopsy directed at the solid tumor component. |
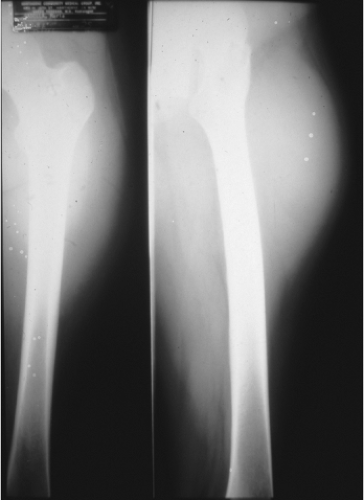 Figure 34.10. Plain extremity radiograph of a high-grade liposarcoma. Films reveal ill-defined soft-tissue density probably arising from the vastus lateralis. |
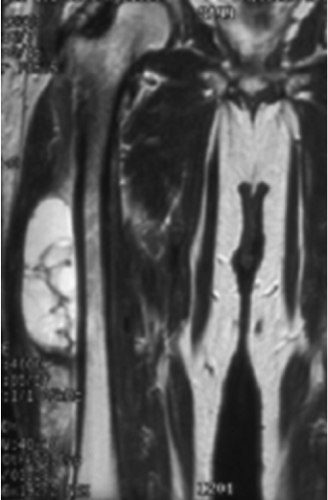 Figure 34.11. Coronal MR demonstrating abutment of a soft-tissue sarcoma to the periosteum of the femur. |
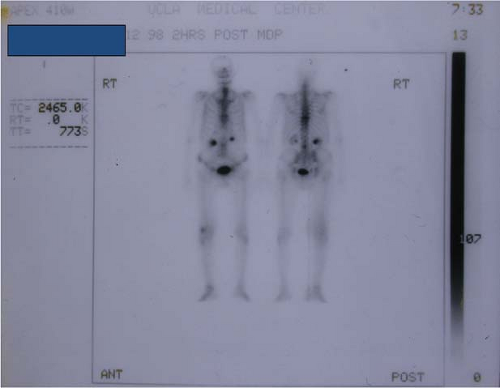 Figure 34.12. Isotope bone scan of a high-grade sarcoma of the distal right thigh. Study demonstrates increased tracer uptake due to periosteal reaction to overlying neoplasm. |
Field Length
Radiotherapy should be directed to those tissues at risk for residual neoplastic cells. Theoretically, the entire compartment is implicated, but there is no proof that routinely
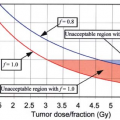
irradiating “origin to insertion” yields significantly better local control rates than a judiciously designed field encompassing the tumor or its resection bed plus a longitudinal margin of uninvolved tissue. The volume at risk for the radiation oncologist certainly includes all tissue manipulated by the surgeon, including drain sites. This volume will likely be larger when patients are treated postoperatively as opposed to preoperatively. Tepper has stressed that the length of the risk volume is not always equivalent to the length of the definitive incision in a postoperative setting (45). A better indication of the field length required for postoperative radiotherapy can be determined from radiodense vascular clips placed by the surgeon at the margins of resection. In practicality, these clips are not always utilized for outline of the resection site. In lieu of formal tumor bed clipping, the postoperative field traditionally extends 5- to 8-cm cephalocaudal to the surgical scar for low-grade tumors and ≥10 cm for intermediate-/high-grade tumors. Mundt et al. have recently challenged these recommendations in a review of postoperatively irradiated patients (46). Those with <5-cm initial, longitudinal margins around the tumor bed and scar had a 5-year local control rate of 30% compared to 93% for those with ≥5 cm (p = 0.0003). There was no significant local control difference, however, between those with initial margins of 5 to 9.9 versus ≥10 cm. This impact of field size applied only to intermediate- and high-grade tumors. No low-grade tumor recurred locally regardless of margins. The local control rate for intermediate grade tumors was 0% with an initial margin <5 cm compared to 92% with larger margins. Local control rates for high-grade tumors were 47% and 92%, respectively. There was no benefit to margins ≥10 cm for either intermediate or high-grade sarcomas compared to 5 to 9.9 cm. Margins of 5 to 9 cm, therefore, appear appropriate for intermediate-grade sarcomas. Five centimeters are sufficient for low-grade sarcoma, although routine adjuvant radiotherapy may not be necessary for a small low-grade tumor affecting a single muscle bundle and amenable to wide myomectomy. There is no need to electively irradiate the draining lymph nodal regions although satisfying field length requirements may unavoidably include these areas. Every attempt should be made to exclude major joint spaces from the radiotherapy beam, especially the weight-bearing hip and knee, while still fulfilling the above field length stipulations. Such protection may not be reasonable and, in some cases, the appropriate field length may be longer than the compartment in question due to the length of the scar (Fig. 34.14).
irradiating “origin to insertion” yields significantly better local control rates than a judiciously designed field encompassing the tumor or its resection bed plus a longitudinal margin of uninvolved tissue. The volume at risk for the radiation oncologist certainly includes all tissue manipulated by the surgeon, including drain sites. This volume will likely be larger when patients are treated postoperatively as opposed to preoperatively. Tepper has stressed that the length of the risk volume is not always equivalent to the length of the definitive incision in a postoperative setting (45). A better indication of the field length required for postoperative radiotherapy can be determined from radiodense vascular clips placed by the surgeon at the margins of resection. In practicality, these clips are not always utilized for outline of the resection site. In lieu of formal tumor bed clipping, the postoperative field traditionally extends 5- to 8-cm cephalocaudal to the surgical scar for low-grade tumors and ≥10 cm for intermediate-/high-grade tumors. Mundt et al. have recently challenged these recommendations in a review of postoperatively irradiated patients (46). Those with <5-cm initial, longitudinal margins around the tumor bed and scar had a 5-year local control rate of 30% compared to 93% for those with ≥5 cm (p = 0.0003). There was no significant local control difference, however, between those with initial margins of 5 to 9.9 versus ≥10 cm. This impact of field size applied only to intermediate- and high-grade tumors. No low-grade tumor recurred locally regardless of margins. The local control rate for intermediate grade tumors was 0% with an initial margin <5 cm compared to 92% with larger margins. Local control rates for high-grade tumors were 47% and 92%, respectively. There was no benefit to margins ≥10 cm for either intermediate or high-grade sarcomas compared to 5 to 9.9 cm. Margins of 5 to 9 cm, therefore, appear appropriate for intermediate-grade sarcomas. Five centimeters are sufficient for low-grade sarcoma, although routine adjuvant radiotherapy may not be necessary for a small low-grade tumor affecting a single muscle bundle and amenable to wide myomectomy. There is no need to electively irradiate the draining lymph nodal regions although satisfying field length requirements may unavoidably include these areas. Every attempt should be made to exclude major joint spaces from the radiotherapy beam, especially the weight-bearing hip and knee, while still fulfilling the above field length stipulations. Such protection may not be reasonable and, in some cases, the appropriate field length may be longer than the compartment in question due to the length of the scar (Fig. 34.14).
 Figure 34.15. Linear accelerator couch at extreme elevation to provide the extended distance necessary to irradiate entire thigh with an isocentric anterior–posterior technique and a single field. |
Extended treatment distances are often required to encompass these long fields. Modern rotational linear accelerators are capable of treating both anterior and posterior fields without turning patients and are strongly recommended for irradiating extremities (Fig. 34.15). If this
equipment is not available, irradiation may be delivered on the treatment room floor with the necessity for turning the patient and individual simulations for each field (Fig. 34.16). Another solution would involve the use of matched fields with an appropriate skin gap calculation. This alternative would leave some superficial tissue, including the incision, undertreated unless the gap is moved periodically.
equipment is not available, irradiation may be delivered on the treatment room floor with the necessity for turning the patient and individual simulations for each field (Fig. 34.16). Another solution would involve the use of matched fields with an appropriate skin gap calculation. This alternative would leave some superficial tissue, including the incision, undertreated unless the gap is moved periodically.
 Figure 34.16. Patient receiving extended anterior distance treatment while supine on the floor. Extended distance posterior treatment will require prone reposition and a separate simulation.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|