ATA [8]
Over age of 45 years with grossly visible extrathyroidal extension at the time of surgery and a high likelihood of microscopic residual disease
Gross residual tumour for whom further surgery or RAI would probably be ineffective
BTA [9]
Gross evidence of local tumour invasion at surgery, presumed to have significant macro-or microscopic residual disease, particularly if the residual tumour fails to concentrate sufficient amounts of RAI
Extensive pT4 disease in patients >60 years of age, with extensive extranodal spread after optimal surgery, even in the absence of evident residual disease
In a study by Farahati et al., 169 patients with pT4N0-1M0 DTC underwent total thyroidectomy, RAI therapy and TSH suppression between 1979 and 1992 in Germany [1]. Ninety-nine patients underwent adjuvant EBRT to the neck, consisting of 50–60 Gy followed by a 6–10 Gy boost to high-risk regions. The planning target volume included the thyroid bed, anterior neck from mastoid or hyoid down to carina, and the supraclavicular regions. The addition of EBRT improved locoregional recurrences (p = 0.004) and distant failures (p = 0.0003).
In an analysis of 382 patients with DTC treated at the Princess Margaret Hospital (Canada) between 1958 and 1985, multivariate analysis revealed that age of >60 years, tumour size of >4 cm, multifocality, postoperative residual disease, lymph node involvement, less extensive surgery and lack of use of RAI were significant predictors of locoregional failure [3]. Whereas EBRT was not found to improve locoregional control or cause-specific survival (CSS) in the entire cohort of patients, a beneficial effect was found for the subgroup of 155 patients with papillary histology and microscopic residual disease with 10-year CSS of 100 % vs. 95 % (p = 0.038) and 10-year local relapse-free rate of 93 % vs. 78 % (p = 0.01) for those with EBRT versus those without, respectively.
More recent reports have also been published examining outcomes of patients treated with EBRT using more modern radiotherapy techniques. In a retrospective analysis from MD Anderson (USA), 131 patients with DTC who received EBRT between 1996 and 2005 were examined [5]. Of these, 96 % had extraglandular disease and 47 % had positive surgical margins. Patients underwent a median 60 Gy using 3-dimensional conformal radiotherapy (3DCRT) or intensity-modulated radiotherapy (IMRT) techniques. With a median follow-up of 38 months, the 4-year locoregional relapse-free survival was 79 %. Multivariate analysis revealed gross residual disease (p < 0.0001) and high-risk histological features (Hurthle cell, tall cell, clear cell, or poorly differentiated features) (p = 0.0021) predicted for increased risk of locoregional relapse.
At Memorial Sloan-Kettering Cancer Center (USA), 76 patients with non-ATC were treated between 1989 and 2006 [4]. Of these, 84 % had DTC histology and 84 % were T4. Treatment included surgery before EBRT in 93 % and RAI in 74 % of patients. EBRT was delivered with a median of 63 Gy using IMRT in 63 % of patients. The treatment field included ‘low-risk’ regions, including cervical lymph node regions II–VI and the upper mediastinum. ‘High-risk’ regions included the thyroid and tumour bed, trachea-oesophageal groove, central nodal compartment and pathologically involved lymph node levels. The 4-year overall locoregional control rate for the entire cohort was 72 %.
Radiotherapy Technique
In relation to the treatment of head and neck cancers (HNCs), IMRT has permitted the development of highly conformal radiotherapy plans, which can sculpt the dose in a concave fashion around important organs at risk, such as the spinal cord, oesophagus, trachea and parotid glands (Fig. 10.1). This was not possible previously by using a 3DCRT technique. Randomized controlled trials have shown that IMRT results in superior salivary gland function and quality of life compared with 2-dimensional and 3DCRT techniques in the treatment of HNCs [10, 11].
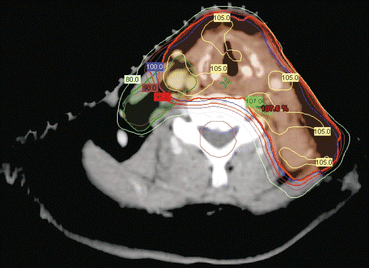

Fig. 10.1
Intensity-modulated radiotherapy plan for thyroid cancer, which shows the ability to generate concave dose distributions that spare organs at risk (e.g. spinal cord)
Studies examining IMRT specifically for the treatment of thyroid cancer are few. However, many of the benefits seen in the treatment of other HNCs would probably apply. In a radiotherapy planning study, Nutting et al. found that IMRT improved planning target volume coverage and reduced the dose to the spinal cord [12]. This would potentially allow dose escalation or minimization of radiation myelopathy after EBRT. Also, in the previously mentioned study of 131 patients treated with EBRT for DTC from MD Anderson, 56 % underwent 3DCRT and 44 % underwent IMRT [5]. The authors found that IMRT was associated with less frequent severe late radiation toxicity compared with 3DCRT techniques (2 % vs. 12 %, respectively).
Radiotherapy Dose and Volume
IMRT is capable of accurately delivering different doses of radiotherapy to separate clinical target volumes (CTVs). The ‘high-risk’ CTV encompasses gross residual disease and positive margins, the tumour bed, central lymph node region, as well as involved nodal regions. The ‘low-risk’ CTV includes uninvolved bilateral cervical lymph nodes (levels II–VI) and superior mediastinal lymph node regions. Supporting evidence suggests that treating larger volumes, including the upper mediastinum, might result in improved disease outcomes [13].
In the author’s practice, the high-risk CTV volume receives 66 Gy in 33 fractions whereas the low-risk CTV volume receives 59.4 Gy in 33 fractions. Other institutions have also used slightly different dose-fractionation schemes with ≤4 dose-levels, depending on the particular high-risk features involved [5].
Using doses of adjuvant EBRT of >50 Gy is supported by clinical evidence. In one study that examined 41 patients with DTC treated between 1988 and 2001 at two UK cancer centres, indications for EBRT included: (i) macroscopic residual disease (56 %), (ii) microscopic residual disease (24 %), (iii) Hürthle cell variant (7 %), (iv) multiple lymph nodes (7 %), and (v) focus of poor differentiation (5 %) [14]. The target volume was from the mastoid to sternal notch, and laterally the junction of the outer and middle-third of the clavicles. Radiotherapy techniques and doses were variable. Doses ranged from 37.5 to 66 Gy over 3–6.5 weeks, and 35 patients (85 %) received at least one dose of RAI. The 5-year local recurrence rates for patients who received <50 Gy, 50–54 Gy and >54 Gy were 63 %, 15 % and 18 %, respectively (p = 0.02 for trend). The authors concluded that doses of at least 50 Gy were required to improve local control [14].
Toxicity
EBRT for thyroid cancer is associated with acute toxicity, including radiation dermatitis, mucositis, oesophagitis, dysgeusia, dysphagia and laryngitis. Late toxicity can include soft-tissue fibrosis, xerostomia, tracheal stenosis, dysphagia and oesophageal stricture. Also, a second malignancy caused by EBRT is a risk. The use of advanced radiotherapy techniques, such as IMRT, can minimize severe toxicity, as mentioned above.
In the Memorial Sloan-Kettering experience delivering a median 63 Gy, acute grade 3 mucositis was present in 18 % of patients and dysphagia in 32 % [4]. IMRT was used in 63 % of these patients. Late, severe (grade 3+) toxicities were generally rare and present in <5 % of patients (grade 3 xerostomia 1 %, grade 3 dysphagia 4 %, grade 4 laryngeal oedema 2 %) [4]. This rate of late toxicity is comparable to that found in the MD Anderson experience in which patients treated with IMRT had a 2 % incidence of late severe toxicities [5].
Anaplastic Thyroid Cancer
Although rare, ATC often presents with rapid and devastating locoregional symptoms, including dysphagia, dyspnoea, haemoptysis and superior vena cava syndrome [15]. Optimal treatment of ATC involves multimodality therapy with surgery, radiotherapy and systemic agents [16]. The ATA has recently published guidelines for the management of patients with ATC [17].
Outcomes after treatment for ATC remain poor. In a SEER (Surveillance, Epidemiology and End Results) analysis of 516 patients with ATC treated between 1973 and 2000, the overall cause-specific mortality rate was 68.4 % at 6 months and 80.7 % at 12 months [18]. Multivariate analysis revealed that predictors of lower cancer-specific mortality were age of <60 years, intrathyroidal tumour, and the combined use of surgery and EBRT.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree