Introduction
Diabetes is considered a cardiovascular (CV) risk equivalent disease. In 1998, Haffner et al. reported that Finnish patients with T2DM have a risk for future major coronary events similar to that of patients with previous myocardial infarction (MI).
1 Other studies have shown that coronary mortality at the time of acute myocardial infarction (AMI) is essentially doubled in patients with diabetes compared with those without diabetes.
2,
3 Moreover, the mortality rates for patients with diabetes who survive an initial MI are doubled for subsequent events when compared to patients who are euglycemic.
4Due to the high risk of both primary events and mortality following onset of coronary heart disease (CHD), intensive prevention of atherosclerosis in all patients with diabetes appears to be a reasonable objective.
5Adult Treatment Panel III (ATP III) defines CHD risk equivalent as the likelihood of developing a major coronary event (myocardial infarct + coronary death) within 10 years of greater than 20%.At current retail drug prices, when 10-year risk for hard CHD events (MI + CHD death) ranges from 10% to 20% per year, statins carry an acceptable cost-effectiveness according to a cost analysis by ATP III.
5Eighty percent of diabetes-related mortality is attributable to the three major forms of macrovas-cular complications, which include CHD, stroke, and peripheral arterial disease (PAD).
6 Table 7-1 shows the predictors of CV mortality in patients with diabetes, and
Table 7-2 displays the features commonly associated with CHD and diabetes.
Roughly 85% of acute strokes are atherothrombotic, and the rest are hemorrhagic (10% pri-mary intracerebral hemorrhage and 5% subarachnoid hemorrhage). The risk of atherothrombotic stroke is two- to threefold higher in patients with diabetes, while the rates of hemorrhagic stroke and transient ischemic attacks (TIAs) are similar to those of the nondiabetic population.
7Predictors of increased mortality among stroke patients with diabetes include (a) severity of hyperglycemia on admission, (b) infarct of the middle cerebral artery, (c) advanced age, (d) presence
of atrial fibrillation, (e) history of congestive heart failure, (f) chronic kidney disease (CKD), and (g) a low Glasgow coma score. Renal failure is a rare cause of death in acute stroke, yet suggests the presence of significant vascular disease. Stroke patients with diabetes also require longer hospital stays when compared to euglycemic individuals. This will inevitably translate into higher overall cost. The longer length of stay might possibly be caused by the difficulties in controlling the blood glucose during the hospital stay.
8,
9

Pathogenesis of Macrovascular Compromise in Patients with Diabetes
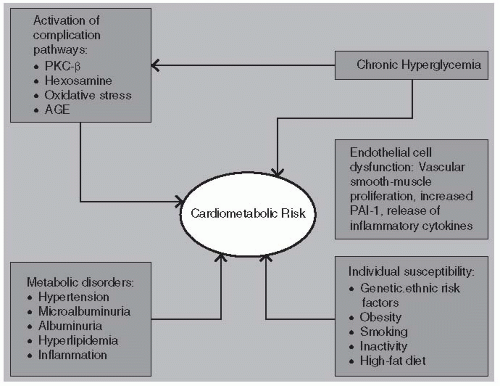
Vascular disease and endothelial dysfunction are accelerated in diabetes due to the multiple metabolic anomalies associated with hyperglycemia (
Fig. 7-1). Damaged endothelial cells produce lower levels of nitric oxide (NO), thereby inhibiting vasodilation. Vascular smooth-muscle proliferation in association with higher circulating levels of plasminogen activator inhibitor-1 (PAI-1) leads to a state of hypercoagulation, increased thrombosis, and progressive atherogenesis. Inflammation within the endovascular environment is accelerated in the presence of hypertension, dyslipidemia, cigarette smoking, and direct endothelial cell damage from cytokines such as tumor necrosis factor (TNF) and C-reactive protein (CRP).
Glucose can react extracellularly in nonenzymatic reactions. One pathologic mechanism shared with microvascular complications involves the glycosylation of protein within arterial wall matrix in a process that induces cross-linking of collagen within the vessel wall, reducing compliance. Direct glycation of low-density lipoprotein cholesterol (LDL-C) prolongs the half-life of these atherogenic lipoproteins, further increasing CV risk. Activation of the protein kinase C (PKC)-β pathway in association with the oxidative stress induced by glycemic variability increases risk of cardiomyopathy.
10,
11 Glycemic variability and oxidative stress appear to be the cornerstone for both microvascular and macrovascular pathogenesis.
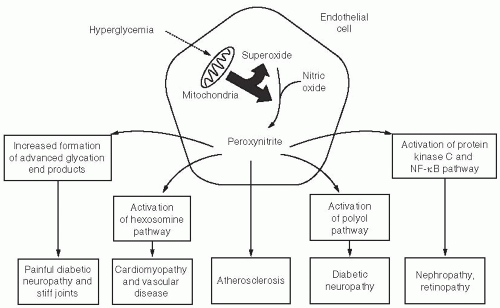
12Why are some tissues (such as neurons or endothelial cells) prone to develop complications, whereas others (digestive cells) appear to be immune to the effects of prolonged exposure to hyperglycemia? The answer may lie in a cell’s ability to assimilate the amount of glucose required as an energy source, before pumping any excess glucose to the outside of the cell. Neurons, nephrons, retinal cells, and endothelial cells are inefficient interstitial transporters of glucose. As glucose levels rise above 180 mg per dL in these “at risk cells,” reactive oxygen species (ROS) form within their mitochondria electron transport chain, triggering a cascade of events leading to microvascular and macrovascular disease.
13 The “downstream” targets of ROS formation include increased activity of PKC, activation of nuclear factor (NF)-κB, collagen synthesis, and cell death.
14 Exposure to blood glucose levels above 180 mg per dL for just 4 hours can cause the downstream effects of ROS to persist for up to 7 days, even if the blood glucose levels are quickly normalized.
15 One can easily understand the biologic link between the extremely high incidence of cardiovascular disease (CVD) and hyperglycemia as shown in
Figure 7-2.
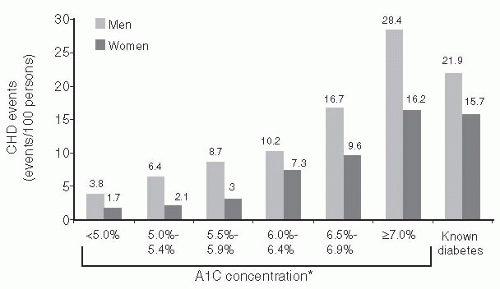
CV risk is increased in individuals who have glucose abnormalities that are considered as being below the diagnostic threshold level for diabetes.
16 The A1C levels of 10,232 patients were assessed from 1995 to 1997. CVD events and mortality rates were subsequently assessed through 2003. As shown in
Figure 7-3, the risk for CVD and mortality increased continuously across the A1C distribution.
As with microvascular disease, improvement in glycemic control can significantly lower the risk of macrovascular complications. The UK Prospective Diabetes Study (UKPDS) demonstrated that each 1% reduction in A1C was associated with a corresponding 14% reduction in the risk of MIs.
17 There was no A1C threshold that targeted the optimal macrovascular risk reduction. Thus, lowering the A1C as close to normal as possible is certainly a desirable target.
The 10-year UKPDS follow-up observed a “legacy effect” of intensive glucose therapy suggesting con-tinued vascular benefits despite loss of glycemic differences from conventional treatment.
18 Reductions in MI and all-cause mortality, which had not been statistically significant in the original trial, became sig-nificant for the intensively treated participants during the post-trial period (
Table 7-3). Thus, the UKPDS follow-up supports initiation of intensive glucose therapy as early as possible for patients with T2DM.
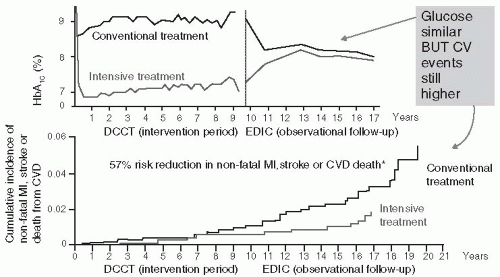
Patients with T1DM who were intensively managed on average for 6.5 years in the Diabetes Control and Complications Trial (DCCT) and then followed for 17 years once the study concluded had a significantly lower rate of CV events than those patients treated with conventional therapy (
Fig. 7-4).
19 Intensive insulin management in T1DM patients reduced the risk of nonfatal MI, stroke
: or death from CVD by 57% and the risk of any CVD event by 42% compared with convention-ally treated DCCT patients. Associated with the prolonged improvement in the A1C, intensively managed patients had lower A1C levels as well as lower incidences of microalbuminuria and albu-minuria, both of which are associated with CVD. Other studies have demonstrated that intensive therapy reduced the progression of atherosclerosis, measured by carotid intima-media thickness (CIMT), as well as the prevalence of coronary artery calcification.
20,
21 The mechanisms responsible for the improvement in outcomes and for the prolonged effects on early intervention remain uncer-tain. Some investigators feel that “metabolic memory” results in long-term beneficial effects on mac-rovascular risk reduction in intensively managed patients with T1DM. Regardless of the protective mechanism associated with improved glycemic control, the risk reduction achieved by initiating intensive therapy in T1DM patients as soon as the diagnosis is made is compelling. Risk reduction achieved with other proven interventions such as lowering cholesterol and BP is far less than those attained by intensively managing patients with diabetes.
Risk reduction for macrovascular disease involves targeting treatment at each one of the meta-bolic abnormalities that coexist with hyperglycemia.
22

Determining Cardiovascular Risk Assessment in Patients with Diabetes
The use of a mathematical model to predict the likelihood of developing long-term diabetes-associated complications would help the physician target specific treatment targets, which should lower the
risk. In addition, global risk assessment models could be useful in educating patients regarding the importance of fine-tuning metabolic therapy or maintaining adherence to a prescribed treatment plan. Risk factor assessment may target two different populations. Patients who have already suffered a diabetes-related complication, such as a stroke or AMI, should be evaluated to determine which interventions may be prescribed to prevent the occurrence of a secondary event. Primary preven-tion strategies are used to delay or avoid an initial event from occurring in high-risk patients such as AMI, stroke, acute coronary syndrome (ACS) (angina), and lower extremity amputation due to PAD.
The major and independent risk factors for CHD are cigarette smoking of any amount, elevated BP, elevated serum total cholesterol and LDL-C, low serum high-density lipoprotein cholesterol (HDL-C), diabetes mellitus, and advancing age (
Table 7-4). Preventive efforts should target each major risk factor. Any major risk factor, if left untreated for many years, has the potential to pro-duce CHD. Nonetheless, an assessment of total (global) risk based on the summation of all major risk factors can be clinically useful for three purposes: (a) identification of high-risk patients who deserve immediate attention and intervention, (b) motivation of patients to adhere to risk-reduction therapies, and (c) modification of intensity of risk-reduction efforts based on the total risk estimate.
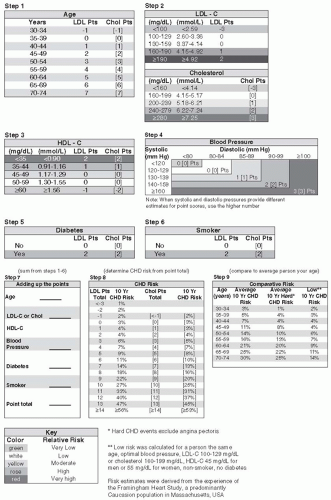
The Framingham Risk Score (FRS) incorporates well-established risk factors such as age, sex, smoking history, blood pressure (BP), total serum cholesterol, HDL-C, and blood glucose (or his-tory of diabetes) into a risk calculation model.
23 However, Framingham tends to underestimate the true 10-year CV risk associated with having T2DM.
24 The initial Framingham cohort included a low baseline prevalence of patients with diabetes while omitting any reference to triglycerides (TGs), an important determinant in CV risk for patients with T2DM.
25 This leads to wide confidence intervals in the overall predicted risk. A wide confidence interval implies that more data should be collected before conclusions may be made in regard to defining specific risk within a given cohort.
The modified FRS (
Fig. 7-5) addresses short- and long-term CV risks in adults with existing CHD. Patients characterized as being “high risk” are predicted to have at least a
2% to 3% yearly likelihood of having a significant cardiac event.
26 The American College of Cardiology recommends aggressive risk reduction for all patients with diabetes as displayed in
Table 7-5 as these individuals have CHD risk equivalent disease.
27Disease risk factors can be defined as measurable biologic characteristics of an individual that precede a well-defined outcome of a disease (such as myocardial infarct), predict that outcome, and
are directly related to the pathogenic pathway. In contrast, nontraditional biomarkers are biologic indicators of a disease process that are involved in the development of the disease, which may or may not be causal.
28 Risk factors identify asymptomatic individuals who have a greater chance of developing a disease compared with the general population. A clinically useful biomarker should meet both of the following criteria: (1) evidence from prospective studies either cohort or random-ized trials across a broad range of populations demonstrating independent prediction of vascular events with reclassification of risk based upon the inclusion of that biomarker and (2) therapies that modify a given biomarker should be available that would otherwise not be used in the at-risk popu-lation. A biomarker that is not useful in predicting disease causality is not considered a risk factor, yet may still elucidate vital information related to disease progression. Biomarkers may also be useful in drug development and measuring therapeutic outcomes.
Table 7-6 lists several novel biomarkers related to inflammation, oxidative stress, and insulin resistance.
The U.S. Preventive Services Task Force (USPSTF) has stated that the use of “high-sensitivity C-reac-tive protein (hs-CRP), ankle-brachial index (ABI), leukocyte count, fasting blood glucose level, periodon-tal disease, CIMT, coronary artery calcification score on electron-beam computed tomography (EBCT), homocysteine level, and lipoprotein(a) [Lp(a)] level are insufficient to screen asymptomatic patients with no history of CHD for primary prevention.”
29 To be fair, the USPSTF recommendation statement clearly says that the critical gap in the evidence for screening with nontraditional risk factors is the lack of infor-mation on subsequent reductions in risk for CHD events in persons identified by the new risk factors.
The Centers for Disease Control and Prevention (CDC) and the American Heart Association (AHA) released a joint statement in 2003 on the use of hs-CRP in assessing CHD risk.
30 Their recom-mendations include the following:
hs-CRP is the CHD inflammatory marker of choice for clinical practice.
Patients with an intermediate (10% to 20%) risk of CHD may benefit from measurement of hs-CRP for evaluation and therapy decisions (this would include all patients with diabetes).
hs-CRP may be useful for estimating prognosis in patients with CHD (including death and recurrent events).
Hyperglycemia is associated with a rise in hs-CRP. Hypertensive patients with T2DM have the highest plasma levels of hs-CRP suggesting that these individuals have an active and on-going inflammatory process perhaps predisposing them to CVD.
31 In 2008, the JUPITER trial was published that showed that statin use (rosuvastatin) in healthy men aged greater than 50 and in healthy women aged greater than 60 with an LDL-C of less than 130 mg per dL and an hs-CRP greater than 2 mg per L decreased the incidence of a first MI by 48% and stroke by 55%.
32 The best primary prevention CV outcomes occurred in patients who attained an LDL-C of less than 70 mg per dL and an hs-CRP of less than 1 mg per L with rosuvastatin.
33 In general, hs-CRP levels may be reduced with the use of statins, β-blockers, or aspirin by 20% to 30%.
34,
35A 15-year longitudinal observational study demonstrated that plasma vitamin D levels less than 13.9 nmol per L in patients with T2DM place them at increased risk for all-cause mortality includ-ing deaths from CVD.
36 Vitamin D appears to mitigate vascular inflammation by affecting foam cell (macrophages which ingest oxidized LDL) formation and signaling in individuals who do not have diabetes.
37Several risk engines are useful in assessing the likelihood of developing heart disease and strokes. The UKPDS Risk Engine (available for downloading at: http://www.dtu.ox.ac.uk/index. html7maindoc=/riskengine/download.html) provides risk estimates and 95% confidence intervals in patients with T2DM not known to have heart disease. A patient’s risk for heart disease and stroke can be calculated for any given duration of T2DM based on current age, sex, ethnicity, smoking status, presence or absence of atrial fibrillation, and levels of A1C, systolic BP, total cholesterol, and HDL-C.
One of the most detailed and patient-friendly global risk engines, Diabetes PHD (Personal Health Decisions), can be accessed as a link through the American Diabetes Association (ADA) Web site (https://www.diabetes.org/phd/profile/start.jsp). Diabetes PHD can be used to explore the effects of a wide variety of health-care interventions (both behavioral and pharmacologic) on the 30-year risk of developing a heart attack, stroke, kidney disease, lower extremity amputation, and DR. Infor-mation that needs to be uploaded into the site includes a detailed health history (MI, stroke, angina, bypass surgery, angioplasty, heart failure, retinopathy, albuminuria, CKD or ESRD, diabetic neuropa-thy, neuropathic ulcer), age, ethnicity, sex, height, weight, lipid levels, smoking history, BP reading, dates of last eye exam, frequency and intensity of exercise, frequency of office visits, A1C, foot exam, presence and level of proteinuria, list of medications, length of time patient has used aspirin, family history of diabetes and/or CVD, and a list of all current medications related to managing diabetes, hypertension, and hyperlipidemia. Diabetes PHD, as powered by a health modeling program known as Archimedes, then calculates the patient’s risk of developing microvascular and macrovascular
complications over the next 30 years. Patients can explore the effects various treatment interventions (both pharmacologic and behavioral) will have on predicted microvascular and macrovascular outcomes using this web site. An updated version of Diabetes PHD is currently being developed by the ADA.

Targeting Optimal Metabolic Control in Patients with T2DM
Data from the UKPDS suggested that improved glycemic control reduced the risk of CVD and deaths as well as all-cause mortality
38 However, three recently published large, randomized, controlled trials (ADVANCE, ACCORD, and VADT) found no evidence that intensive glycemic control had a major effect on CV outcomes (
Table 7-7).
39,
40,
41ACCORD identified an increased risk for death from CV causes and total mortality associated with intensive glucose control, thereby providing little guidance on how to manage metabolic control in high risk patients with type T2DM. The lessons learned from these landmark studies are listed in
Table 7-8.
Based upon the studies mentioned above, health-care providers should focus their efforts on combining elements of lifestyle modification, glycemic control, which minimizes hypoglycemia and weight gain, BP reduction, and optimal lipid lowering to reduce macrovascular complications in patients with T2DM.

Macrovascular Complications in Patients with T1DM
Although less studied than in patients with T2DM, macrovascular complications can impact those individuals with T1DM at a younger age (less than 40 years), be more diffuse, have a greater accelerated course to end-stage outcomes than observed in the general population. The Pittsburgh Epidemiology of Diabetes Complications study demonstrated that both men and women had similarly increased risk of premature coronary artery disease (CAD), although risk factors, such as renal impairment appeared to be gender specific.
42 As with T2DM, risk factors for macrovascular complications in T1DM include hypertension, smoking and dyslipidemia, insulin resistance, African American race, and disease duration.
43,
44Genetic variation may play a major role in predicting which patients may be predisposed to CHD. In the Pittsburgh EDC study, patients who carried the haptoglobin (Hp)2-2 genotype had a twofold greater risk of CHD than those expressing the Hpl-1. A major limitation for the management of CHD in T1DM is the lack of a validated risk engine for this patient population. Therefore, genetic studies may prove valuable in future risk assessment in patients with T1DM.
45Long-term follow-up of the intensively treated patients in the DCCT suggest that treatment to AlC targets less than 7% in the years soon after the diagnosis of the disease is associated with reduction in macrovascular risk.
19 The establishment of “metabolic memory,” appears to warrant treating hyperglycemia as soon as possible, for as long as possible, as low as possible, as safely as possible, and as rationally as possible in all patients with diabetes.
46