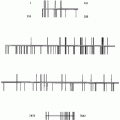
Cervarix
Gardasil
Gardasil 9
HPV coverage
16, 18
6, 11, 16, 18
6, 11, 16, 18, 31, 33, 45, 52, 58
Manufacturer
GlaxoSmithKline
Merck
Merck
Producing cells
Trichoplusia ni (Hi 5) insect cell line infected with L1 recombinant baculovirus
Saccharomyces cerevisiae (baker’s yeast) expressing L1
Saccharomyces cerevisiae (baker’s yeast) expressing L1
Adjuvant
Aluminum hydroxyl-phosphate sulfate
Aluminum hydroxide, 3-O-deacylated-4’-monophosphoryl lipid A
Aluminum hydroxide, 3-O-deacylated-4’-monophosphoryl lipid A
Administration
Intramuscular
Intramuscular
Intramuscular
Injection schedule
0, 1, 6 months
0, 2, 6 months
0, 1–2, 6 months
Indications for use (HPV-associated diseases)
Cervical cancer and precancer and adenocarcinoma in situ
Women 9–26 years: cervical, vulvar, vaginal, anal cancer and precancer; genital warts.
Men 9–26 years:
anal cancer and precancer; genital warts
Women 9–26 years:
cervical, vulvar, vaginal, anal cancer and precancer; genital warts.
Men 9–26 years:
anal cancer and precancer; genital warts
Both of the first-generation prophylactic vaccines have exhibited excellent safety and effectiveness. However, neither of the vaccines has shown efficacy against a prevalent infection (Schiller et al. 2012). There has been no standard assay for assessing immunogenicity in HPV VLP vaccine trials, making direct comparisons difficult. However, regardless of the assays used, studies have demonstrated antibody responses that are strong and durable up to 6–8 years (Brotherton et al. 2016; Einstein et al. 2014; Roteli-Martins et al. 2012; Olsson et al. 2007).
One of the key issues in HPV vaccination is the most optimal timing for the HPV vaccines to provide maximal protection of HPV-naïve individuals (Malagon et al. 2012). Recent studies have confirmed that HPV is present in the semen, placenta and in the newborns (Rintala et al. 2004, 2005; Sarkola et al. 2008; Laprise et al. 2014; Skoczynski et al. 2015; Chisanga et al. 2015). Data from our Finnish Family HPV cohort indicate that persistent oral HPV infections among newborn are predicted by mother’s placental HPV positivity (Koskimaa et al. 2012). This implicates that the timing of HPV vaccination needs to be re-evaluated. In already infected women, HPV vaccination will not alter the course of their genital tract infection although it induces higher HPV IgG antibody titers. During pregnancy, there might be more efficient transplacental transfer of maternal HPV antibodies to prevent the newborn to contract HPV infection during delivery. The natural history of the first HPV exposure is not elucidated yet, but there is a possibility that the first site of HPV entry is the oral mucosa (Syrjänen et al. submitted; Rintala et al. 2005).
2 Natural History of HPV in Head and Neck Region
The prevalence and natural history of HPV infection in the head and neck region has not been well established yet. Our prospective Finnish Family HPV cohort on HPV dynamics among family members has shown that HPV infection can be acquired vertically by the newborn before, during and after delivery, and HPV can be found both in the oral and genital mucosa (Sarkola et al. 2008; Rintala et al. 2004, 2005; Syrjänen et al. submitted). The site of the first HPV infection may be through oral mucosa more likely than the genital mucosa. These infections of the newborns create cell-mediated immunity (CMI) against HPV, including the T memory cells (Koskimaa et al. 2014, 2015). In adult life, HPV prevalence seems to be lower in oral mucosa than in the genital region (Chung et al. 2013). Studies using mouth rinses/gargles have found HPV prevalence ranging between 0.9 and 7.5 % (Edelstein et al. 2012; Gillison et al. 2012; Pickard et al. 2012; Smith et al. 2007; Summersgill et al. 2001). With mucosal swab samples and using more sensitive HPV detection methods, HPV prevalence is considerably higher (Kero et al. 2012, 2014; Rautava et al. 2012a, b).
Understanding the natural history of HPV infection in its whole, and the events leading to head and neck infection and cancer in particular, is the critical questions to be solved before conclusions can be drawn whether the current HPV vaccine strategies of adolescents also protect against HNSCC.
3 HPV Antibodies in Saliva
Oral cavity has its own specific environment including saliva. Saliva has secretory immunoglobulin A (sIgA) which is important in the control of infectious agents on oral mucosal surfaces. A study showed that low levels of sIgA could make the individual more susceptible to oral HPV infection (Gonçalves et al. 2006). Saliva could be a noninvasive testing alternative to serum testing for HPV antibodies (Cameron et al. 2003). Women with cervical neoplasia showed significantly more sIgA for HPV16 than women with no cervical neoplasia (Marais et al. 2006). However, opposite results were reported from Costa Rican women by Kemp and co-workers (2012). Immunoglobulin levels could be more site specific than compartmental between mucosal sites (Passmore et al. 2007). Women with a persistent oral HPV infection showed higher levels of salivary IgG and lysozyme than women with no oral HPV infection (Haukioja et al. 2014). In this study, smoking was a risk of persistent oral HPV infection. Prophylactic HPV vaccination has also been shown to induce neutralizing antibodies in saliva (Handisurya et al. 2016). An approach of mucosal HPV immunization has also been explored, because mucosal surfaces are the site of HPV infection (Kichaev et al. 2013). In Sweden, it was shown that after gradual introduction of public HPV vaccination during 2007–2012, between 2013 and 2014, when 73 % of the women were HPV vaccinated, but not necessarily before their sexual debut, oral HPV prevalence had dropped to 1.4 % as compared with 9.3 % in 2009–2011 (p < 0.00001) (Grun et al. 2015).
4 Prevention of HPV-Associated Head and Neck Cancer with Vaccination
HPV-associated HNSCC, more specifically the incidence of oropharyngeal cancer, is dramatically increasing in industrialized countries (Gooi et al. 2016; Marur and Forastiere 2016), but there are no data on vaccine efficacy against oropharyngeal HPV infections (Chaturvedi et al. 2011). Table 2 summarizes the prevalence of HPV in HNSCCs derived from systematic reviews including meta-analyses. Because the most common oncogenic HPV genotypes (HPV16 and HPV18) found in the head and neck malignancies are the same as in cervical cancer, the question is: do prophylactic HPV vaccines effectively prevent HPV-related head and neck pathologies (Beachler et al. 2015; Herrero et al. 2013). However, HNSCCs may also harbor HPV genotypes that are not so common in genital malignancies (Rautava et al. 2012a, b). Moreover, Gardasil has demonstrated protection against genital warts and penile/vulvar/vaginal/anal neoplasia in addition to cervical cancer (Schiller et al. 2012; Garland and Smith 2010; Goldstone et al. 2013). Vaccination efficacy is lower when the subjects have already an ongoing HPV infection (Lu et al. 2011). These data suggest that vaccinating the males protects them also against most vaccine HPV-type-related anogenital diseases, which has led to registration and use of these vaccines for males in some countries (Palefsky et al. 2011; Giuliano et al. 2011). For men, immunization through the oral mucosa might be even more important than for women, because the largest mucosal area of men is in the oral cavity (Marais et al. 2006). Quadrivalent vaccination also induces neutralizing antibodies in oral mucosal fluids (Handisurya et al. 2016). Cost-effectiveness analysis has shown that HPV vaccination for boys aged 12 years could be a cost-effective strategy for the prevention of oropharyngeal carcinoma (Graham et al. 2015).
Table 2
Systematic reviews including meta-analysis of head and neck cancers
Reference | Sample size | Cancer | HPV positivity | Population |
|---|---|---|---|---|
Gama et al. (2016) | 7347 | Laryngeal SCC | 1830 (25 %) | Global |
Shaikh et al. (2015) | 7280 | Head and neck carcinoma | 36 % | Asian Pacific region |
Zhang et al. (2014) | 3429 | Esophageal cancer | HPV16 prevalence 0.381 (95 % CI: 0.283, 0.479) | China |
Aboqunrin et al. (2014) | 3649 | Head and neck cancers | 40 % | European |
Hardefeldt et al. (2014) | 132 studies | Esophageal SCC | 24.8 % | Global |
Syrjänen and Syrjänen (2013) | 492 | Sinonasal SCC | 133 (27.0 %) | Global |
Syrjänen (2013) | 10,234 | Esophageal SCC | 3135 (30.6 %) | Global |
Mehanna et al. (2013) | 19,368 (5396 oroph, 13,972 non-oroph) | Oropharyngeal/non-oropharyngeal SCC | Oroph 47.7 % Non-oroph 21.8 % | Global |
Termine et al. (2008) | 4852 | Head and neck SCC/oral SCC | 34.5 %/38.1 % | Global |
5 Therapeutic HPV Vaccines and HNSCC
Therapeutic HPV vaccines could eliminate preexisting lesions and infected cells. Before using HPV vaccine in the treatment of a certain patient, there should be established HPV status and histological results. However, today there is no agreed standard method for HPV testing. Most therapeutic vaccines have been developed with HPV16, against its oncoproteins. Targets for therapeutic intervention are HPV E6 and E7 oncoproteins since they are expressed at all levels of the HPV-infected epithelium and play a role in the induction and maintenance of HPV-related cancer.
There are trials ongoing with several different molecular targets. A phase I dose escalation trial of MAGE-A3- and HPV16-specific peptide immunomodulatory vaccines GL-0810 and GL-0817 showed T cell and antibody responses in a majority of HNSCC patients (n = 16) (Zandberg et al. 2015). One consequence of E6/E7 over-expression in HPV-associated oropharyngeal cancer is a strong expression of the cellular protein p16INK4a (Reuschenbach et al. 2016). A recent report of phase 1/2a first-in-human trial on HPV DNA vaccine against cyclin-dependent kinase inhibitor p16INK4a showed induction of cellular and humoral immune responses in advanced tumors without severe toxicities (Reuschenbach et al. 2016). This study with 26 patients included seven HNSCCs. With a mouse model, local irradiation and Shiga Toxin B-based HPV vaccination for treatment of HNSCC have shown promising results and are approaching early-phase clinical trials (Mondini et al. 2015). In another study with mice, intradermal DNA vaccines forming E7 recombinant retroviral virus-like particles (pVLP-E7) cured mice with already established tumors when combined with toll-like receptor (TLR) 7 and TLR 9 agonists (Lescaille et al. 2013). Also the PD-1:PD-L1 pathway has shown a possibility of therapeutic blockade analyzing tissue samples from tonsillar carcinoma patients (Lyford-Pike et al. 2013). For comparison, quadrivalent vaccination effectiveness in low-risk HPV-induced respiratory papillomatosis has either shown no effect in children (Hermann and Weckx 2016) or prolonged the intervals between the surgical interventions (Hocevar-Boltezar et al. 2014).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree