Keywords
Adoptive immunotherapy, dendritic cells, electropermeabilization, gene delivery systems, gene editing, gene silencing, gene transfer, hematopoietic, lentiviral vectors, LTR, mesenchymal stem cells, mitochondrion gene therapy, pseudotyping, retroviral vectors, RNAi, RRE, stem cell engraftment, transpozons, WPRE
Introduction
Let’s indulge ourselves and state the obvious: Not all cells are created equal. What is even worse from the viewpoint of the gene therapist is that cells in vivo are also not equally accessible nor equally suitable for gene therapy. In addition, control of dose distribution in vivo is complicated, making it potentially more difficult to achieve clinical success. Ex vivo approaches are favored because of the ability to specifically select target cells and modify them in a controlled environment prior to their reintroduction into subjects. Although the methodology for gene transfer has had to overcome many hurdles , the current vectors and ex vivo methods being utilized are bearing positive results in the clinic.
Gene Delivery Systems
Physicochemical Methods
As far back as 1944, Avery demonstrated that inheritance is DNA mediated and purified naked DNA can penetrate the cell membrane and transfer genes (cells can be transduced). This is an excellent proof of concept, but the reality is that the naked DNA, even in the form of plasmids, is a rather inefficient agent of gene delivery. A series of physical DNA delivery systems have been devised throughout the years with some success. Ballistic or “gene gun” DNA delivery technology uses DNA-coated gold or polymer particles and uses the kinetic energy of the particles accelerated by compressed air or fluids to penetrate the cell membrane and deliver the DNA payload . Although up to 20% transfection efficiency has been achieved under optimized single cell layers , the technology seems to be more appropriate for gene delivery into tissue that has substantial depth, such as the cornea, skin, or muscle cells . Ballistic gene delivery is likely to find application in the field of DNA vaccination rather than ex vivo cell therapy due to the overall inefficiency of the method.
A wide array of cationic liposomes, including targeted liposomes and liposomes using helper lipids are being developed as gene delivery systems because they have tantalizing promise in the area when safety measures are highly coveted and capacity to carry large chunks of DNA is prized . The lineup also includes oligoarginine–PEG–lipid particles , archosomes based on lipids isolated from various bacteria (Archaea) , exotic lipoplexes, polyplexes, and nanoparticles (reviewed by Pathak et al . . The goal of the ongoing development is to improve the otherwise suboptimal transduction performance of these methods.
Electropermeabilization (or electroporation) is another physical method to render the cell membrane permeable to DNA and facilitate integration. The method relies on delivery of strong electric pulses (up to several hundred volts), which increases the transmembrane potential, disturbs the membrane bilayer, and induces nanopores. The advanced electroporator systems currently available allow precise control of the procedure, which improves cell survival as well as the transient gene delivery rate .
Transposons
The “moveable genetic elements” or transposons were originally discovered in corn by Barbara McClintok in 1948. Currently, several transposon vectors are being developed. The one that may have the highest medical relevance is “Sleeping Beauty” (SB), a Tc1/mariner-like transposon . SB encodes a synthetic transposase enzyme that recognizes SB-specific inverted repeats and mediates precise cut-and-paste transpositions in fish, murine, and, more important, human cells. Integration requires a TA dinucleic motif, of which approximately 200 million are available in the human genome, so the insertion potential is high as well as efficient. The SB technology combines the advantages of retroviral (efficient integration) vectors as well as the disadvantages of the naked DNA (low efficiency of cell entry). Early studies indicated that transposons have high mutagenic potential, are able to randomly disrupt important genes, and can relocate within the genome (estimated rate of one event per several months). However, the current preclinical and clinical trials indicate a lower genotoxicity rate than expected, and it remains a promising system worthy of further exploration .
Viral Vectors
In contrast to the physicochemical gene delivery methods and transposons, viral vectors rely on the endowed properties of viral particles to (A) encapsulate the genetic material, which is double-stranded RNA in the case of retrovirus-based vectors and double-stranded DNA in the case adeno and adeno-associated vectors; (B) attach to cells through virus receptors; (C) enter the target cell using their fusogenic ENV proteins (enveloped retroviral) or endocytosis (nonenveloped, adeno, and adeno-associated vectors); and (D) inject their payload (RNA/DNA) into the cytoplasm of the cells.
At this point, two classes of viral vectors can be differentiated by their fate within the cell. The adeno and adeno-associated vectors are DNA-based and remain mostly extrachromosomal elements, whereas the retroviral (RNA-based) vectors reverse transcribe the RNA into DNA and actively target the nucleus and subsequently integrate into the genome using integrase, an enzyme carried within the viral vector particle and associated with the vector’s ribonuclear complex.
The advanced form of viral vectors, the adenoviral vectors, have been used in more than 300 clinical trials, but their usage is limited because they carry and express highly immunogenic proteins and because of limited stability of the gene expression. Also, despite a large body of work, their true clinical value remains to be confirmed .
The adeno-associated vectors are considered rather safe because the virus itself is considered nonpathogenic and has a preferred (and therefore predictable) integration site on chromosome 19 with a negligible frequency of random integration events. The chromosome 19 integration capability has been removed from the advanced versions of the vector by deleting rep and cap genes. The adeno-associated vectors have shown some clinical success and have been used in approximately 80 clinical trials, but due to their small size, they can only carry small payloads (~4 kB), which limits their usability to small genes. This limitation has led to intensive work-in-progress to increase the vector’s payload capacity . Nonetheless, adeno-associated virus vectors appear particularly suited for direct gene delivery of smaller genes and interfering RNA (RNAi) into nondividing cells such as those that constitute the central nervous system or muscle.
Gamma-Retroviral Vectors
Although the first success of ex vivo cell therapy was generated by γ-retroviral gene transfer into SCID-X1 patients , it also drew attention to a set of unexpected side effects, namely the integration of the therapeutic vector, a γ-retroviral derivative, into the “LIM domain only 2” (LMO2) proto-oncogene, resulting in its activation and constitutive cytokine production. Subsequent chromosomal rearrangements led to severe lymphoproliferative disorder in three patients, one of whom died and the remaining two responded to the therapy and benefited from the cell therapy (see the review by Bushman ). The events shed light on the inherent feature of genus Retroviridae, which were known to be involved in oncogenesis via their unique ability to cause genotoxicity by integration into proto-oncogenic sites . A series of studies was initiated to better understand the genotoxic risk of oncoretroviral and the other major retroviral-derived vectors, the lentiviral vectors. Naldini and colleagues compared the relative genotoxic potential of oncoretroviral vectors and lentiviral vectors. Using the Cdkn2a −/− mouse transplantation model, which is susceptible to a broad range of cancer-triggering genetic lesions, they found that oncoretroviral vectors triggered dose-dependent acceleration of tumor onset, whereas tumorigenesis was unaffected by lentiviral vectors, despite a higher integration load and robust expression of lentiviral vectors in all hematopoietic lineages. The results of these studies have been confirmed by others and indicate a favorable safety profile for lentiviral vectors , especially when used with less powerful promoters.
Lentiviral Vectors
Lentiviruses are distinct members of the Retroviridae family of viruses. Lentiviral vectors have been developed with HIV-1 , HIV-2 , feline immunodeficiency virus (FIV) , equine infectious anemia virus (EIAV) , and simian immunodeficiency virus (SIV) . An important feature of lentiviral vectors is their low genotoxicity compared to that of oncoretroviral vectors, as reviewed previously. Current clinical experience with lentiviral vectors supports these experimental studies. To date, no oncogenic events have been observed in human clinical trials using lentiviral vectors . However, clonal expansion and dominance of hematopoietic progenitors have been reported in a clinical trial in which hematopoietic stem cells were genetically modified with a lentiviral vector that expressed the beta-globin gene for the treatment of thalassemia . Although the mechanisms for clonal dominance remain unclear, it appears that low transduction efficiencies may actually increase the probability for clonal events because of the potential for oligoclonal engraftment in the hematopoietic stem cell population . These findings suggest that vector design, purity, and transduction methods are important considerations when designing clinically relevant studies and for interpreting their data, especially in genotoxic-sensitive cell types such as stem cells. It is worth noting that genotoxicity appears to affect primarily stem cells and not somatic cells. For example, T cells transduced with oncoretroviral vectors are highly resistant to oncogenic transformation .
Lentiviral vectors have become the delivery platform of choice for ex vivo gene therapy due to their large packaging capacity, low genotoxicity, and ability to efficiently transduce dividing and nondividing cells.
A very detailed molecular design of the lentivirus vectors has been reviewed elsewhere . Typically, lentivectors are generated by transcomplementation, a process that separates the essential components of HIV (the genes encoding Gag-Pol, Rev, and Env) into separate plasmids, which lack the packaging signal and therefore can never end up in a packaged vector unless they appear in a recombinant sequence . The components (Tat, Vif, etc.) responsible for pathogenicity by upregulation of transcription and export of genomic RNA to the cytoplasm have been successively removed from the constructs. The potential for a recombination event is minimized, and for all practical purposes avoided, by carefully editing the genes and using codon degeneracy to reduce the chances of recombination with the wild-type virus. These separate plasmids are used to co-transfect a packaging cell, typically HEK293, along with a payload plasmid that carries the packaging signal necessary for starting the envelope formation and encapsidation of the mRNA that carries the payload gene(s) as well as the 5′ and 3′ long terminal repeats (LTRs) necessary for integration into the transcriptionally active regions of the host chromosomes. Packaging is a delicate process, which ensures that with the RNA, appropriate tRNA lys , protease, integrase, and reverse transcriptase enzymes are carried by the vector with the packaging elements necessary for successful cell entry, reverse transcription of vector RNA to DNA, transport of that DNA into the nucleus, and the permanent integration of the DNA into the host chromosome. The ENV gene encodes the protein GP160; this is cleaved into trimer-forming GP120, which appears as spikes decorating the vector particle; and GP41, which carries a transmembrane region and a carboxy-terminal subdomain that interacts with the nucleocapsid within the envelope. The amino-terminal domain has a fusogenic domain that facilitates cell entry by fusing the outer membrane of the vector with the cell membrane. A region further down from the amino-terminal region also binds to GP120, which in turn binds to primary HIV-1 receptors on the target CD4 + of T lymphocytes. If left unmodified, this property would significantly limit the usability of the lentivectors because very few cell types can be directly infected by HIV-1. Pseudotyping overcomes this limitation and permits targeting to any mammalian cell.
Pseudotyping essentially replaces the original HIV ENV gene with a corresponding molecule from other viruses and carries over the cell-targeting specificity (i.e., the tropism of the virus) and obviates some of the safety concerns related to GP120 . The list of successful pseudotypes and cell tropism is rather lengthy and growing. The most successful pseudotype so far uses the ENV from vesicular stomatitis virus (VSVg) that broadens the tropism practically to all cell types in the brain, kidney, liver, and others. It extends to mesenchymal (stem) cells, even those in the nondividing (resting) state . Filovirus ENV pseudotypes shift the tropism to a more limited set of cells—the airway epithelial and endothelial cells . Baculovirus GP64 and hepatitis C virus E1 and E2 pseudotypes redirect the vectors toward liver cells targeting their respective receptor, CD81 (tetraspanin) . Rabies virus ENV has been shown to efficiently retarget the vectors to neuronal and RD114 ENV pseudotyped lentivectors show preference for the hematopoietic cellular compartment cells . However, some applications require targeting a specific cell type, which is not necessarily covered by the available pseudotypes listed previously. In these cases, new targeting methods have been developed to further tighten the tropism of lentiviral vectors by coexpressing cell-specific coreceptors that recognize one of the cell type-specific markers.
The payload plasmid components providing the backbone for the transfer vector in the early HIV vectors were composed of a 5′ LTR, followed by a major splice donor site, a packaging signal site encompassing the packaging signal components of the 5′ region of Gag (necessary for high-efficiency packaging and high vector titer), and a deletion of the rest of the gag gene. Deletion of the U3 region from the 3′ LTR promoters also became possible by constructing genes relaying on their own promoter(s) instead of the native LTRs. The latest generation of lentiviral vectors carry an additional safety element, the self-inactivating LTR (SIN lentivectors , which replaces the LTR with an HIV-independent promoter from cytomegalovirus (sCMV). In these vectors, the LTRs are modified in a way that upon integration they lose their intrinsic promoter ability, reducing genotoxic potential. In addition, the irreversible changes that occur during integration diminish the ability to mobilize after integration and to recombine with other elements to form a full-fledged, replication-capable virus . The formal proof of increased safety is still lacking, ironically, because of the inability to create and detect RCL-capable viruses from lentivector-treated cells , indicating that this risk is mainly theoretical.
The removal of RRE and the associated splice donor and acceptor elements results in significant loss of transduction efficiency of the vector , whereas adding a 100-nucleotide central polypurine tract (central DNA flap) restores the transduction efficiency by improving the reverse transcription and nuclear transport efficiency . The woodchuck hepatitis virus transcriptional regulatory element (WPRE) is another widely used regulatory element added to the lentivector backbone to stabilize the transcription transgene mRNA levels and improve transgene expression . However, an open reading frame of the oncogenic WHV-X element has been found within the native WPRE sequence , so the sequence has been modified to remove the translation start site .
Further optimization continues to improve the safety of lentivectors, such as isolating the integrated vector DNA to prevent translation beyond the vector boundaries by adding isolating elements. However, the insulators have proven to be genotoxic in some instances, and no proof has emerged that such isolators are truly needed . Gene switches such as Tet-On and -Off have been added to subsequent generations of lentivectors and proven to be highly functional, operating with very low leakage . The Cre-Lox system has also been successfully implemented in the lentiviral context, allowing high-efficiency engineering and sophisticated, site-specific recombination techniques, including the delivery and irreversible switching by small hairpin RNA (shRNA) expression , a tool extensively used in gene function analysis .
As mentioned previously, the lentiviral vectors have the unique advantage of being able to stably transduce dividing and nondividing cells . In contrast to oncoretroviral preintegration complexes (PICs), the lentiviral PIC has the capability of active transport into the nucleus through nuclear pore complexes in an ATP-dependent manner , essential for efficient transduction of nondividing cells. What makes lentiviral vectors distinctly useful is their ability to stably transduce a wide variety of mammalian cell lines and primary cells with high efficiency . Moreover, lentiviral vectors can accommodate larger transgenes (up to ~10 kB) compared to when oncoretroviral vectors are used .
Ex Vivo Genetic Modification of Cell Substrates
In general, the genetic manipulation of the isolated cell substrates falls into a few categories. Existing genes can be silenced, downregulated, modified, repaired, or edited.
Gene Silencing with RNA Interference
Gene silencing by small RNAi is based on duplex formation between the mRNA and a short complementary microRNA (miRNA) or small inhibitory RNA, each having the ability to interfere with protein synthesis and downregulate the expression levels of the targeted protein. A major problem with RNAi technologies is the short half-life and delivery of the RNAi. This can be resolved using vectors encoding artificial genes with appropriate miRNA sequences that can be integrated into the host cell DNA and efficiently transcribed into primary miRNA that utilizes the natural intracellular processing by microprocessor complex formation with drosha to form shRNA in the nucleus. The exported miRNA is subsequently cleaved by Dicer and produces the complex forming inhibitory RNAi. The process is rather complex, but it efficiently blocks protein expression that may be incomplete but readily achieves significant reduction, which is adequate for gaining insight into the function of the targeted protein and efficient enough for phase I and II clinical trials, although no therapeutic use has been approved by the U.S. Food and Drug Administration. It is interesting to note that mesenchymal stem cells (MSCs) are capable of secreting cholesterol-rich phospholipid microparticles encapsulating miRNA and therefore have the potential to facilitate intercellular communication and act as regulatory agents in their microenvironment . Davidson and McCray provide an excellent review on the biogenesis and clinical applications of small RNA compounds.
Hematopoietic or general pluripotent stem cells are often selected targets for RNA interference-based interventions, and one of the promising efforts deals with creating artificial virus-resistance genes and virus-resistant somatic cells. Preventing HIV-infection by reconstituting the immune system with such stem cell-derived virus-resistant progeny has been used as a model system with significant clinical relevance . The idea is that an efficient HIV infection requires virus entry through the CD4 surface antigen and one or more virus coreceptors, among which CCR5 has been shown to play an essential role in the case of R5 tropic viral strains involved in primary HIV infection. Clinical data indicate that CCR5 deficiency or certain mutations in this co receptor protect the infected individuals from the onset of full-blown AIDS, and the hope is that the artificial knockdown of CCR5 using gene therapy and RNAi will achieve similar protection . The relative inefficiency of CCR5 suppression remains a significant issue, but major improvement and complete knockdown of CCR5 have been achieved with somewhat longer (28 bases instead of 23) shRNA .
Ex vivo gene therapy research is taking full advantage of the shRNA techniques by characterizing the subtle, and not so subtle, changes induced by individual gene knockdowns. It is a long-held view that mechanical stresses and mechanical characteristics of stem cells, as well as the microenvironment, can affect stem cell proliferation and differentiation. Lentiviral vectors are excellent and efficient targeting tools for these stem cells, even resting ones, and can deliver the shRNA without causing major changes and stress that would otherwise change the stem cells. Chowdhury et al . studied the spreading response of MSCs and showed that myosin II, F-actin, Src, or CDC42 were essential for cell spreading, and changes in the mechanical characteristics (“softening”) of the stem cells led directly to the downregulation of the OCT3/4 gene. This indicates the possibility that small mechanical events may affect the embryo and developing tissues and even transplanted stem cells.
Another area of efficient use of viral vectors and RNAi technology in ex vivo stem cell research is the production of transgenic embryos that carry knockdown genes. Production of transgenic embryos is highly efficient, and if the fertilized egg is transduced at a single cell stage, the entire germline is affected, or partial chimerism can be achieved if multicellular embryos are treated with lentiviral vectors. An example of such a study is that by Wang et al . , in which they showed that the knockdown of RunX1 in embryonal tissues and MSCs by lentiviral vector-delivered RNAi blocked chondrogenesis in limb buds. The technique has been shown to be very efficient for transgenesis: As high as 44% average rate of germline transmission can be achieved , providing a new source of gene-modified MSCs. A comprehensive review of the use of naturally occurring regulatory miRNA technology in MSC research by Guo et al . . indicated that stem cells have discrete and distinct expression profiles that can account for intrinsic stem cell properties such as self-renewal and pluripotency, a property that can no longer be overlooked by experts dealing with MSCs. The accumulating data indicate that the progenitors and terminally differentiated mesenchymal cells can be tracked and defined by function-related miRNAs in addition to the already established sets of surface markers. The miRNAs already identified affect osteogenic differentiation, chondric differentiation, adipogenic differentiation, myogenic differentiation, neuronal differentiation, wound healing, and replicative senescence. These advances open a wide array of possibilities to direct the differentiation patterns of the stem cell population temporarily by using nonintegrating lentiviral vectors that are automatically lost from dividing cell populations and lead to the natural disappearance of control signal after a few cell divisions but potentially give a push to the original stem cell population to develop in a preferred direction.
Extensive progress has been made with regard to the elucidation of the Hedgehog signaling pathway in MSCs using RNA interference delivered with lentiviral vectors. The data suggest that at least some of the elements indeed act through the regulatory miRNA network by downregulating the cellular miRNA levels. However, the data also suggest significant off-target effects of the RNAi molecules and indicate that we are a long way from the potential clinical use of the elucidated networks .
Hu et al . devised an ingenious method to prepare the brain for traumatic interventions (surgery, extensive stem cell transplantations, etc.) by downregulating the cerebral matrix metalloproteinase 9 (MMP9) using lentivectors and MMP-9 shRNA 2 weeks before the trauma. The knockdown of MMP-9 with the shRNA proved to be an effective way to preserve the blood–brain barrier, and they achieved significant reduction of brain infarction volumes, brain water content, and Evans blue/IgG extravasation (measure of edema formation) as well as a reduction in the neurobehavioral deficit in their rat brain trauma model , implying a potential for improved protocols for traumatic brain interventions needed for more extensive types of intracranial stem cell implantations.
Gene Editing Using Zinc Finger Nucleases Encoded within Lentiviral Vectors
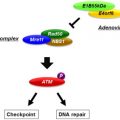
Zinc finger nucleases (ZFNs) have the remarkable ability to (A) bind to a specific location in the double-stranded DNA, break the double-stranded DNA at that specific location and, if an endogenous repair template is provided, initiate homology-directed repair, restoring the integrity of the newly edited double-stranded DNA. As their name implies, there is a specific DNA-binding part of this class of enzymes that consists of a tandem repeat of DNA-binding zinc finger motifs, hence the DNA-binding specificity and a catalytic domain, FokI. For DNA cleavage to occur, FokI has to dimerize, one on the sense and the other on the antisense strand, while the zinc finger domains attach to the right target half site and the left target half site. Upon binding, a nick with a 5′ overhang is initiated by FokI between the target sites, and the homology-directed DNA repair mechanism is activated. What makes this configuration useful is that the spacer between the two target half sites can be several hundred or even thousands of base pairs long, and by providing a template for the activated repair mechanism, a novel DNA sequence of equal length can be introduced into the DNA; see reviews by Carroll and others .
Fundamentally, two factors determine the efficacy of the DNA editing or repair that the technology allows. The first is the specificity of the zinc finger binding, which also determines the length of the spacer and the proper specificity and uniqueness of the binding site and allows the minimization of the off-target effects that may be introduced by similar sites far away from the desired and targeted locus . Major efforts are being made to tailor the ZFNs for particular applications and improve the selectivity by successfully engineering the DNA-binding specificity of the binding domain . The second factor is the efficient delivery of the ZFNs and the template DNA by vectors. Early attempts relied on retroviral vectors, adeno and adeno-associated vectors, and even baculovirus and lentiviral vectors. A conversion rate as high as 50% can be achieved with lentiviral delivery in a variety of cell lines and human embryonic stem cells compared with the earlier best rates of 18% with other methods in human and other species .
Gene Transfer
As mentioned previously, lentiviral vectors provide a very efficient method for generating transgenic embryos, significantly reducing the need for the generation of a high number of embryos to establish new sources of gene-modified stem cell lines, embryonic or other . The lentiviral technology is able to deliver a payload of 6–8 kB very efficiently, but payloads of 10 kB can be handled, and delivery of payloads of 12 or 13 kB is possible at a cost of lower efficiency. This payload-carrying capacity allows the delivery of very large genes, such as the gene-encoding blood clothing factor VIII, a 2351-amino acid-long protein together with its stabilizer, the von Willebrand factor (2813 amino acids in its native form) simultaneously, or, one may need to use a domain-engineered and shortened version of both. Similarly, it can be used to deliver all three chains of an IgM molecule in a single, tri-cistronic complex. The implication is that the lentivector system has sufficient payload capacity to deliver a number of relevant genes together with several supporting molecules envisioned for highly complex gene therapy scenarios currently outside the scope of monogenic gene therapy as practiced today. In the future, it may be used to target diseases with multigene disorders such as high blood pressure, arthritis, or diabetes.
Cell Substrates for Ex Vivo Cell Therapy
Selecting the proper cell type and matching it to the disease/malfunction and the related molecular pathways is an issue for which the solution lies mainly in the area of stem cell research and lineage-specific differentiation. The issue is far from trivial because the known 23,000 or so human genes allow a possible expression pattern of 2 23,000 just in the case of binary expression (on/off) but even orders of magnitude higher as the expressions are not necessarily binary. It indicates a fundamental deficiency in our understanding of how the cells function as stable building blocks of healthy tissues . For example, although bone marrow transplantation has a long history the gene expression profiles that account for engraftment, differentiation, and repopulation are just starting to be understood with high-throughput technologies. A vast amount of experience has amassed regarding the possible complications and the inherent complexity of outcome in terms of tissue compatibility and engrafting success .
T Cells
Efficient gene transfer into T lymphocytes may allow not only successful treatment of several rare monogenic diseases, such as severe combined immunodeficiency , but also the development of novel therapeutic strategies for cancer . Ex vivo transduced T cells were used in cancer clinical trials for suicide gene (HSK-Tk) transfer in allogeneic T cells as part of allogeneic bone morrow transplantation, as well as for direct attack on tumor cells, when they are modified with tumor-specific T cell receptors or chimeric antigen receptors (CARs) (reviewed in Frecha et al . .
For ex vivo transduction by γ-retrovectors or lentiviral vectors, T cells are purified from patient apheresis by density gradient centrifugation and, in some cases, also by immunoaffinity separation using magnetic beads (MACS) . Murine retroviruses cannot transduce nondividing cells; therefore, T cells have to be stimulated by cytokines at the start of the transduction process. Interleukin 2 (IL-2) and anti-CD3 antibodies were initially used for this purpose . However, stimulation with polystyrene beads bearing anti-CD3 and anti-CD28 antibodies proved to be more efficient .
Lentiviral vectors can transduce nondividing cells; however, resting T lymphocytes are refractory to gene transfer with lentiviral vectors . Activation of these cells, causing G0 to G1b transition of the cell cycle, is required to relieve the blocks in gene delivery. Moreover, it has been reported that inducing the resting T cells to enter into the G1b phase of the cell cycle without triggering cell division could render them permissive to transduction with HIV-1 vectors . Thus, CD3/CD28 costimulation of T cells greatly enhanced their transduction by lentiviral vectors .
Transduction of T cells with lentiviral and γ-retrovirus vectors is usually performed for 1 or 2 days with one or two additions of the vector. To increase transduction efficiency, plates are often coated with retronectin . After transduction, T cells are expanded to obtain at least 10 10 cells per patient .
Genetic modification of T cells by ex vivo transduction to redirect antigen specificity is an attractive strategy compared to the lengthy process of growing T cell lines or CTL clones for adoptive transfer. The approaches currently studied in several laboratories involve the genetic transfer of chimeric antigen receptors and high-affinity natural T cell receptors. CARs (or T-bodies) are artificial T cell receptors that combine the extracellular single-chain variable fragment (scFv) of an antibody with intracellular signaling domains, such as CD3ζ or Fc( ε )RI γ . When expressed on T cells, the receptor bypasses the need for antigen presentation on MHC because the scFv binds directly to cell surface antigens. This is an important feature because many tumors and virus-infected cells downregulate MHC, rendering them invisible to the adaptive immune system. The high-affinity nature of the scFv domain makes these engineered T cells highly responsive even in the case of low antigen densities on cancer cells. In addition, new CARs are relatively easy to produce from hybridomas. Because one can redirect both CD4 and CD8 T cells, the T-body approach to immunotherapy represents a near universal “off the shelf” method to generate large numbers of antigen-specific helper and cytotoxic T cells.
The key to successful adoptive immunotherapy strategies appears to consist of (1) the modification of selected T cells with desired functional characteristics and (2) obtaining therapeutically effective numbers of these cells without compromising their effector functions or their ability to engraft within the host . The success of the CD3/CD28 costimulation protocol developed at the University of Pennsylvania was demonstrated in the clinic when a patient was successfully treated for chronic lymphocytic leukemia by autologous CAR-modified T cells .
Hematopoietic Stem Cells
Hematopoietic stem cells (HSCs) are excellent targets for gene therapy due to the relative ease with which they can be manipulated and their ability to repopulate the entire hematopoietic system for the life of a patient. The HSC must be permissive for transduction by the proposed vector; the vector genome must be efficiently maintained in daughter cells; and transduction must not impair the ability of the HSC to renew, differentiate, or expand. To date, only the retroviral vectors such as the γ-retroviral, lentiviral, and foamy vectors have fulfilled these criteria.
Historically, transduction of stem cells in large animal models including nonhuman primates and in humans has proved challenging. A significant effort from several research labs was required to develop an acceptable process for clinical transduction of HSCs (reviewed in Nienhuis ). The strategies used by scientists include optimization of the cytokine combination as well as the use of a fragment of fibronectin, retronectin, to co-localize vector particles and target cells during transduction as reported by the laboratory of David Williams .
The use of alternative envelope proteins to pseudotype vector particles, including that derived from gibbon ape leukemia virus (GALV) , resulted in improved transduction efficiency of human hematopoietic cells. More efficient pseudotyping of lentiviral vectors has been achieved using stem cell factor (SCF) and a endogenous retroviral glycoprotein, RDTR. These RDTR/SCF lentiviral vectors outperformed RDTR lentiviral vectors for transduction of human CD34(+) cells (hCD34(+)) .
Dendritic Cells
The ability of dendritic cells (DCs) to provide all three signals required for T cell activation makes them an ideal (cancer) vaccine platform. Several strategies have been developed to enhance and control antigen presentation, costimulation, and cytokine production (reviewed in Boudreau et al . ). For DC modification strategies, the most extensively used signal is the sorting sequence of lysosome-associated membrane protein-1 (LAMP-1). The LAMP-1 sorting signal has been coupled to gene modification vectors, including vaccinia virus encoding pp65 , retrovirus coexpressing HPV16 E7 (98), and through mRNA electroporation with carcinoembryonic antigen , human telomerase reverse transcriptase (hTERT) , and Mage-A3 .
CD40–CD40L is the costimulatory receptor/ligand pair whose expression has been most often enhanced for the purpose of improving DC function. Ligation of CD40 on DCs is normally provided by activated CD4 + T cells . This interaction is the mechanism through which CD4 + type 1 T helper (Th1) cells provide help in generating primary CD8 + T cell responses. This mechanism has been mimicked by engineering DCs to express CD40L through transduction with adenovirus , lentivirus vector , and vaccinia virus or through mRNA electroporation .
DCs are capable of priming both proinflammatory and regulatory/suppressive T cell responses based on the complement of costimulatory receptors (or lack thereof) that they express. The downregulation of suppressive molecules in DCs is therefore an attractive approach for generating therapeutic immunity against cancer. Although many molecules qualify for this purpose (reviewed in Mao and Wu ), only a few have been investigated by genetic modification of DCs. Surface molecules that have direct suppressive effects on T cells are also attractive targets for silencing. To date, two surface molecules have been evaluated for this purpose: the Notch ligands and DC-derived immunoglobulin receptor 2 (DIgR2). The expression of Notch ligands (Delta1, Jagged1, or Jagged2) has been shown to deliver suppressive signals to T cells . Knockdown by small interfering RNA (siRNA) in human DCs leads to enhanced interferon-γ (IFN-γ) production in allogeneic mixed lymphocyte reaction .
In addition to cognate antigen recognition and costimulation, DC-derived soluble factors create a critical third signal to condition the immune environment. To facilitate development and maintenance of Th1 signaling after vaccination, DCs can be modified for constitutive production of Th1 cytokines and chemokines. IL-12p70, produced by DCs after stimulation, initiates Th1 polarization by inducing upregulation of tumor necrosis factor-α, IFN-γ, IL-2, and IL-18 from neighboring leukocytes (reviewed in Del Vecchio et al . ). DCs transduced using recombinant adenovirus carrying IL-12 demonstrate increased antigen presentation and costimulatory molecule expression, and they induce increased numbers of activated T cells .
Preclinical models of DC-based cancer vaccines provided significant optimism for translation to clinical application. Protocols for deriving DCs from CD14 + and CD34 + monocytes are established, and DCs are well tolerated in phase I clinical trials . Genetic modification of DCs is safe and sufficient for delivery of TAAs, costimulatory molecules, and the environmental signals. The use of DNA, mRNA, or viruses to introduce TAAs allows for endogenous expression and processing of full-length proteins, including tumor antigens and immune response factors. Moreover, chemokine, cytokine, and costimulatory molecule expression can be made continuous by delivering the relevant genes under the control of constitutive promoters.
Mesenchymal Stem Cells
Stem cells, including MSCs, are very close manifestations of Plato’s imagery of the shadows on the cave wall because they are difficult to study outside their intimate interactions with their microenvironment . Nevertheless, ex vivo , MSCs are a stromal cell type, possessing the following characteristics and markers: plastic adherence in cell culture, specific surface antigen expression of CD105 (+), CD90 (+), CD73 (+), CD34 (−), CD45 (−), CD11b (−) or CD14 (−), CD19 (−) or CD79a (−), HLA-DR1 (−), and multilineage in vitro differentiation potential (osteogenic, chondrogenic, and adipogenic) . However, this definition would neatly exclude CD34 + HSCs, and one could also argue that the HSCs are just a specialized subclass of the mesenchymal progenitors . Another subset of MSCs that express hyaluronan (CD44), an adhesion molecule important for stem cell homing , would also be excluded, but their perivascular equivalents could be considered to be true MSCs . It becomes even more complicated if we include the results of (stem) cell reprogramming, when more or less differentiated cell types are regressed into less differentiated, pluripotent cell types , providing us with a never-ending stream of novel biomarkers, more often represented by whole proteome analysis . The biomarkers and criteria for properly characterizing the particular stem cell populations will likely be determined in the future, but currently there is a functional definition that provides a firm conceptual handle on the idea of cell plasticity and which cells are the prominent representatives . The plasticity indicates the ability of mature or not fully differentiated cells to differentiate into novel cell types, or more accurately, it describes the existence of cells specialized into becoming progenitors of differentiated cells while sustaining their own type and maturation level. There is no doubt that we will find the appropriate placement for the specialized subtypes as well as the proper and practical placement of some of the induced pluripotent cells in the realm of MSCs.
Regardless of their classification, mesenchymal cell lineages are good candidates for stem cell therapy , gene therapy , cell reprogramming , delivery of bioactive molecules , and tissue engineering . In addition, a number of new issues are arising from the results describing the importance of stem cells in inducing and sustaining the malignant phenotype and the potential therapeutic targeting of a wide range of the elusive cancer stem cell types . Expression of proteins that can modulate their biology or therapeutic properties enormously expands their utility for therapy.
Mitochondrion-Specific Targeting
An interesting twist in the story of cell therapy is the attempt to redirect the gene of interest from the nucleus to organelles. The mammalian mitochondria are the prime targets of interest for those medically inclined due to the large number—more than 300—of the known mutations causing metabolic diseases and disorders directly affecting the mitochondrial genome (see http://www.mitomap.com ). Niazi et al . detail the progress in this emerging field. The idea behind this effort is that although the vast majority of proteins in the mitochondria are imported from the genes residing in the nucleus, the mitochondria still encode 13 polypeptides of the oxidative phosphorylation pathway, 22 tRNA species, and 2 ribosomal RNA—all in a compact 16.5-kB genome.
The first successful mitochondrial manipulation can be traced to Nagley et al . in 1988, but it was slow to advance until Guy et al . developed a mammalian model system, and subsequently a series of different gene-transfer methods have been validated . Regardless, the progress was slow and intermittent. Nevertheless, it was pursued by a number of cutting-edge scientists. Electroporation, ballistic (kinetically driven, DNA-coated particles), and import pathway targeting (protofection) have been tested, and each has been proven to transfer genes successfully into individual purified mitochondria or, as with mitochondriotropic liposomes, to target mitochondria residing inside the targeted cells (reviewed in Niazi et al . ).
Engraftment with Ex Vivo Genetically Modified Cells
The immunological aspects of cell therapy are usually mild or negligible because often the option of autologous cell transfer is available or there is time to screen properly matched donors and the expressed proteins are native human proteins, with proper glycosylation and conformation. In specific cases, however, such as type I diabetes in which the underlying cause of the primary disease is autoimmunity that destroys the β cells or certain autoimmune types of hemophilia, the native human protein is a well-defined antigen, and patients may respond to it vigorously, rejecting the transplant. In these cases, an active immunotherapy is necessary, and only through some forms of immunotherapy can the immune system be rebalanced to ensure the survival of the remaining β cell population. These techniques include cell ablation, competitive decoy autoantigens, reduced cell activation, and autoantigen introduction that enables the survival of the graft . In the case of hemophilia A, the active immune response may be avoided by using recombinant FVIII lacking the particular antigenic epitopes, replacing native human FVIII with porcine FVIII or its domain-engineered variants .
One promising immunotherapeutic intervention is the use of a limited graft-versus-cancer reaction when the transplanted allogeneic immune cells mount a vigorous immune reaction against the MHC antigen-expressing cancer in the host . However, in a number of patients, the graft-versus-host disease can ensue . To limit the progress of the GVHR, suicide genes may be inserted into the donor cells prior to transplantation, and at the onset of the GVHR the donor cells are decimated by chemical means .
Major limitations to gene therapy using ex vivo transduced cells are the limited number of cells involved and the low gene transfer efficiency. In addition, the inability of most therapeutic genes to confer any selective advantage on the gene-corrected cells results in unacceptably low engraftment and ultimately to failure of cell therapy.
One approach to enrich for gene-modified cells in vivo is to include in the retroviral vector a drug-resistance gene, such as the P140K mutant of the DNA repair enzyme O 6 -methylguanine-DNA methyltransferase (MGMT) . This approach can also be used in the clinic to protect the transduced cells against toxic effects of chemotherapy, especially in the treatment of glioblastoma . Successful protection of cells by this approach was shown in clinical trials. HSCs transduced with retroviral vector, expressing mutant MGMT (P140K), efficiently engrafted in three patients with glioblastoma. All three patients surpassed the median survival for glioblastoma patients with poor prognosis, with one patient alive and progression-free more than 2 years after diagnosis. Thus, transplanted P140K-expressing hematopoietic stem and progenitor cells are chemoprotective, potentially maximizing the drug dose that can be administered .
Stem cells, which are one of the favorite targets of ex vivo gene therapy, are rare: It is estimated that only 1 in 10,000 cells in normal tissues belongs to this self-renewing cell population. It is also known that for the cells to survive, they require a supporting microenvironment, and even if they survive, they do not differentiate appropriately in the absence of a proper microenvironment . Because stem cells spend much of their time in the resting state, sitting in the stabile microenvironment that is inaccessible to productive engraftment by the transplanted (stem) cells, only a fraction of transplanted cells can home successfully to these niches and function as expected . To improve the engraftment rate, the recipient can be pretreated, so as to cause activation of the resting stem cells and mobilize them, thus multiplying the number of the available microenvironmental niches for the transplanted cells .
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree