Thomas B.L. Kirkwood
Evolution Theory and the Mechanisms of Aging
The question “Why does aging occur?” calls for answers at the level of proximate physiologic mechanisms and at the level of ultimate evolutionary origins. This chapter provides an understanding of why aging has evolved and examines what evolution theory can tell us about the types of mechanisms we might regard as prime candidates to explain senescence.
Evolution theory is well recognized as a powerful tool with which to inquire about the genetic basis of the aging process.1–4 Although human aging has its roots long ago in our past, the study of its evolution can throw important light on key present-day challenges. For example, a range of population-based studies, including one based on genealogic analysis of the entire population of Iceland, has shown consistent evidence for a genetic contribution to human longevity.5 There is growing interest in knowing how many and what types of genes are likely to be involved in this heritability.6,7 There is also interest in human genetic disorders such as Werner syndrome and Hutchinson-Gilford progeria, which are characterized by acceleration of many aspects of the senescent phenotype (see Chapter 11).
Before addressing questions about the evolutionary origin of aging, it is important to be precise about how the term aging is to be understood. In this chapter, aging is defined as “a progressive, generalized impairment of function, resulting in a loss of adaptive response to stress and in a growing risk of age-related disease.” The overall effect of these changes is summed up in the increase in the probability of dying, or age-specific death rate, in the population.
This definition of aging—in terms of a mortality pattern showing progressive increase in age-specific mortality—allows comparisons to be made, even among species in which the detailed features of the aging process may differ markedly. In phylogenetic terms, aging is widespread but by no means universal.8–12 The fact that not all species show an increase in age-specific mortality indicates that aging is not an inevitable consequence of wear and tear. On the other hand, the fact that very many species do show such an increase is evidence that the evolution of aging has occurred under rather general circumstances.
Evolution of Aging
Theories on the evolution of aging seek to explain why aging occurs through the action of natural selection. The decline in survivorship, which is often also accompanied by a decline in fertility, means that there is an age-associated loss of Darwinian fitness that is clearly deleterious to the organism in which it occurs. Natural selection acts to increase fitness, so it is at once clear that selection should be expected, other things being equal, to oppose aging. Thus, the challenge to evolution theory is to explain why aging occurs, in spite of its drawbacks.
Programmed, or “Adaptive,” Aging
It is sometimes suggested that despite its disadvantages to the individual, aging is beneficial and even necessary at the species level, for example, to prevent overcrowding.13,14 In this case, genes that actively cause aging might have evolved specifically to program the end of life in the same way as genes program development.
The difficulty with this view is that there is little evidence that intrinsic aging serves as a significant contributor to mortality in natural populations,15 which means that it apparently does not play the adaptive role suggested for it. The theory also embodies the questionable supposition that selection for advantage at the species level will be more effective than selection among individuals for the advantages of a longer life. Aging is clearly a disadvantage to the individual, so any mutation that inactivates the hypothetical adaptive aging genes would confer a fitness advantage and, therefore, the nonaging mutation should spread through the population unless countered by selection at the species or group level. Conditions under which this so-called group selection can work successfully are highly restrictive,16 especially when there is selection in the opposite direction acting at the level of the individual. Briefly, it is necessary that the population be divided among fairly isolated groups and that the introduction of a nonaging genotype into a group should rapidly lead to the group’s extinction. The latter condition is necessary to provide the selection between groups that might, in principle, counter the tendency for selection at the level of individuals to favor the spread of nonaging mutants. Although theoretical special cases have been constructed that might permit the selection of genes to cause aging, it appears unlikely that the necessary conditions will be met with sufficient generality to explain the evolution of aging.17
Selection Weakens With Age
An observation of central importance to the evolution of aging is that the force of natural selection—that is, its ability to discriminate between alternative genotypes—weakens with age.15,18–21 Because natural selection operates through the differential effects of genes on fitness, its discriminatory power must decline with age in proportion to the decline in the remaining fraction of the organism’s lifetime expectation of reproduction. This is true whether or not the species exhibits aging.
The attenuation in the force of natural selection with age means inevitably that there is only loose genetic control over the later portions of the life span. For this reason, it has been suggested that aging might be due to an accumulation of mutations in the germline, which potentially are deleterious but are not expressed, or that produce no phenotypic effect until late in life.15
The idea is that if deleterious mutations are expressed so late that most individuals will already have died from some other cause, such as predation, even though the genes involved have the potential to cause harm, they will be subject to very little selection against them. Over the generations, a large number of such genes might accumulate. These would cause aging and death only when an individual is removed to a protected environment, away from the hazards of the wild, and so would live long enough to experience their negative effects.
A stronger version of this theory was proposed by Williams,18 who suggested that because of the declining force of natural selection with age, any gene that conferred an advantage early in life would be favored by selection, even if the same gene had deleterious effects at older ages. Such “pleiotropic” genes could explain aging. The decline in the force of natural selection with age would ensure that even quite modest early benefits would outweigh severe harmful side effects, provided the latter occurred late enough.
Disposable Soma Theory
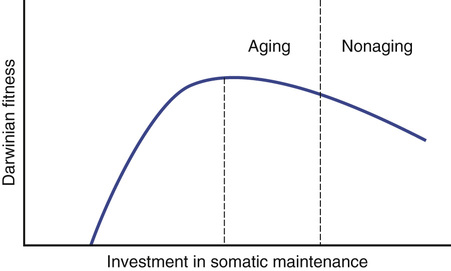
The disposable soma theory1,4,21–23 explains aging by asking how an organism should allocate its metabolic resources best, primarily energy—on the one hand, keeping itself going from one day to the next and, on the other hand, growing its body and producing progeny to secure the continuance of its genes when it has itself died. No species is immune to hazards such as predation, starvation, and disease. All that is necessary by way of maintenance is that the body remains in sound condition until an age after which most individuals will have died from accidental causes. In fact, a greater investment in maintenance is a disadvantage because it eats into resources that in terms of natural selection, are better used for reproduction. The theory concludes that the optimum course is to invest fewer resources in the maintenance of somatic tissues than are necessary for indefinite survival (Figure 5-1). The result is that aging occurs through the gradual accumulation of unrepaired somatic defects, but the level of maintenance will be set, so that the deleterious effects do not become apparent until an age when survivorship in a wild environment would be extremely unlikely.
Comparison of Evolutionary Theories
The adaptive program theory is in a category of its own, and support for this theory is weak; it will not be considered further in this chapter. The disposable soma and pleiotropic genes theories are adaptive in the sense that aging is the result of positive selection for aspects of the organism’s life history, but the essential difference is that aging itself is not adaptive, but is a negative trait, that arises only as a byproduct or tradeoff of some other benefit. The late-acting deleterious mutations theory assumes an essentially neutral evolutionary process, with the accumulation of mutations reflecting the inability of natural selection to maintain tight control over the later portions of the life span.
Among the nonadaptive theories, there is a common strand—namely, that old organisms count less. This is not due to any implicit assumption of frailty or obsolescence, which would render the theories circular, but to the simple mathematics of mortality. Even if old organisms retain exactly the same vigor as young ones, to the extent that old and young are physiologically indistinguishable, the fact that each cohort becomes numerically attenuated with age means that the selection force weakens. The nonadaptive theories are not mutually exclusive. Therefore, aging might in principle be due to a combination of any of them.
As regards the nature of gene action, the disposable soma theory is the most specific of the evolutionary theories because it suggests not only why aging occurs, but also predicts that the genetic basis of aging is to be found in the genes that regulate levels of somatic maintenance functions. Neither the pleiotropic genes theory nor the late-acting deleterious mutations theory is specific about the nature of the genes involved.
Genetics of Life Span
This section discusses the genetics of life span, first from the point of view of interspecies comparisons. That is, it will ask “Why do species have the life spans they do?” It will then look at intraspecies variation and heritability of life span. Finally, there is a brief discussion of human progeroid syndromes, such as Werner and Hutchinson-Gilford progeria, as models of genetically accelerated senescence.
Species Differences in Longevity
In addition to explaining why aging occurs, evolution theory must also account for differences in species life spans. This raises basic questions about the genetic control of aging: specifically, how many genes are involved, and how are these modified by selection to produce changes in life span?
For each of the nonadaptive theories, the generality of the selection forces involved suggests that multiple genes will be implicated. If there is a very large number of independent genes causing aging, however, the life span may be slow to change, because modifying a single gene may have little effect by itself, and the probability of simultaneous independent modifications will be low. This suggests that a reasonably small number of primary genes are responsible for aging, or that there exists some mechanism for coordinate regulation.
The evolution of increased life span is most readily explained if it is assumed that an adaptation occurs that results in a general lowering of the accidental (age-independent) death rate. In the late-acting deleterious mutations theory, this may result in new pressure to eliminate or postpone the deleterious gene effects. In the pleiotropic genes theory, the balance between early benefit and late cost may be shifted in favor of reducing the harmful effects on late survival. In the disposable soma theory, there may be selection to adjust the optimum investment in maintenance to a higher level.
Variation Within Species
The variability in life span observed within a species or population clearly owes much to chance, but there is a significant heritable component as well.5 Martin and colleagues3 have applied the terms public and private to denote genetic factors related to aging that may be specific to individuals or shared across a population (perhaps even across species). Late-acting deleterious mutations are strong candidates for private genes because the fate of such alleles is determined largely by random genetic drift. Public genes are more likely to be those that arise through tradeoffs. In particular, the genes involved in regulating mechanisms of somatic maintenance are likely to be public genes of considerable importance. Although these genes are public in the sense that all individuals have them, there may nevertheless be variations within a population in the precise levels at which these functions are set. These variations in setting may in turn be the cause of genetic variations in life expectancy.
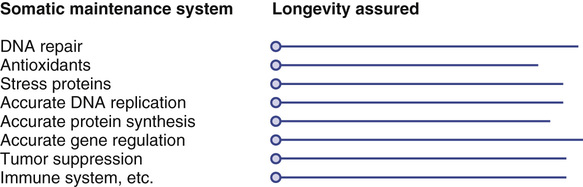
As predicted by the disposable soma theory, the level of individual somatic maintenance systems should be set high enough so that the organism remains in sound condition through its natural expectation of life in a wild environment, but not much higher than this, or resources will be wasted. Numerous maintenance systems operate in parallel to preserve viability (Figure 5-2). Depending on the levels at which they are set, each maintenance system can be thought of as assuring a given span of life (see also Cutler24 and Sacher25 for earlier discussions of the concept of longevity assurance). When any one of these critical mechanisms has exhausted its potential for ensuring longevity, which happens because the accumulated defects threaten survival, the organism is liable to die.