and Winfried G. Rossmanith2
(1)
Lenzkirch, Germany
(2)
Ettlingen, Germany
During the evolution of life, singled-celled organisms developed into multicellular ones and these multicellular organisms became plants or animals. When animals got organs with diverse functions, they had to invent communication between these to establish homeostasis. The devices of this communication are nerves and hormones.
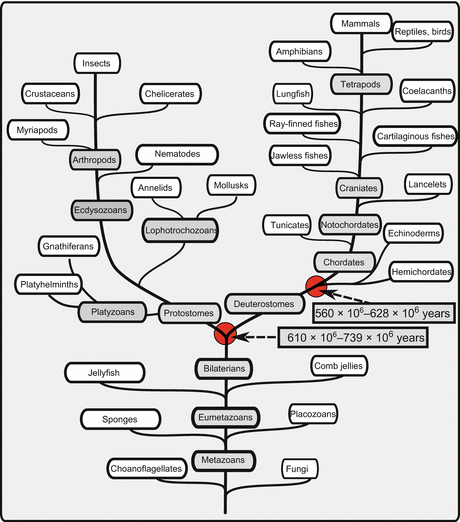
As shown in previous chapters, the biochemical roots of the endocrine systems are beyond the kingdom Metazoa. Potentially steroidogenic enzymes have been found in bacteria, as well as in plants. The roots of the “tree of life” are buried below thick sediments. For Fig. 13.1 we have used the poster of Westheide and Rieger (2007) as a reference. The sketch accompanying the fact sheet on invertebrate neuropeptides is based on information in this poster. Figure 13.1 contains two numbers which indicate the periods where separation of protostomes from deuterostomes and chordates from hemichordates or echinoderms occurred. Scientific names are listed together with common names in the Appendix.
For the text that follows, we assume that the presence of a homologous gene in two species means that the common ancestor already had this gene. If fish and humans have a gene with the same function, then this gene was present when amphibians separated from the fish lineage. If the fruit fly expresses the same cytochrome P450 (CYP) enzyme, then this gene was already present when the lineages giving rise to arthropods and chordatas separated. Such an assumption is based on the sequence analysis of many proteins, especially that of CYP. If a function in two species is due to a structurally similar protein and a gene with a related sequence, then there are better reasons to accept segregation from a common ancestor than a multiple recreation with stunning similarity.
The evolution of the endocrine system as described in humans dates to the beginning of life: cholesterol was already made by protozoans. The last intermediate of cholesterol biosynthesis, 7-dehydrocholesterol (Fig. 6.4, 18) , is isomerized to vitamin D3 by UV light. This reaction is as old as 7-dehydrocholesterol biosynthesis. Thus, we suspect that vitamin D3 has been made for billions of years.
In contrast to these very old molecular origins, the control of calcium homeo-stasis by vitamin D3 is much more recent. As long as metazoans lived in the ocean, the calcium concentration in the body fluid was determined by the calcium concentration in the ocean: about 1 mM.
The presence of a molecule in a species at the beginning of evolution does not argue for an ancient function and action of this hormone as they are found today in humans. Hanke (1970) argues that compounds did exist before they acquired an endocrine function.
Most interestingly, freshwater fish species as well as all land-living animals from amphibians on regulate their serum calcium levels to the calcium concentration of saltwater, as do humans, although the ocean has not been their natural environment for the last 100 million years. For the maintenance of the saltwater calcium levels in blood, freshwater fish and land-living animals have developed parathormone . In fish, parathormone-related peptides are made in corpuscles of Stannius, and in humans they are made in the parathyroid gland. It is possible that parathormone is the last hormone appearing in the evolution of the endocrine system.
In tunicates, iodine is fixed using mucopolysaccharides. Starch, however, can fix iodine without coupling iodine covalently. The development of thyroid peroxidase, which oxidizes iodide to elementary iodine, which is then transferred to the tyrosine residues of thyroglobulin and where one ring is transferred to another iodinated tyrosine residue, is not in any way related to fixing iodine with mucopolysaccharides and mucus. Phagocytosis of the iodine-containing colloid and the enzymatic digestion which gives rise to thyroxine might have been possible in ancestors of fish. The thyroid peroxidase and thyroxine release, however, first occurred in fish, including saltwater fish.
13.1 Division of Labor
Certain eukaryotic single-celled organisms are able to adapt their movement to an external signal and thus to swim/drift in the direction of a food source. The nature of such a signal inducing a directed movement is not known. From the time when single-celled organisms aggregated to multicellular aggregates with assigned roles, intraorganismal communication to balance offer and demand was established. Nerves and neurosecretory cells served for these purposes. The simplest metazoans known, cnidarians belonging to coelenterates, express precursor proteins giving rise to neuropeptides. Nerves in sensu stricto or ganglia have been found only in later animals, molluscs, or annelids. The development of nerves and neuropeptides—among other—distinguishes metazoans (multicellular animals) from plants and fungi.
13.2 Evolution of Neuropeptide Hormones
Since earliest animals expressed neuropeptides, this characteristic has been common throughout the animal kingdom. Neuropeptides normally1 arise as translational products of specialized neurosecretory cells where precursor proteins are successively processed by cell-type-specific enzymes, prohormone convertases, endopeptidase, peptidylglycine alpha-amidating monooxygenase , and if an N-terminal glutaminyl residue is present, glutaminyl cyclase , where mature neuropeptides are stored in granules and where these granules are fused to the cell membrane on interaction of a ligand with a membrane receptor, inducing a rise of intracellular calcium concentration which triggers vesicle–membrane fusion.
The catalog of so-called FMRFamide peptides starts in cnidarians (see Fig. 13.1). In insects—for example, in cockroaches—there is a leucosulfakinin (EQFEDYGHMRFamide) or a leucomyosuppressin (pEDVDHVFLRFamide) (first identified in Leucophaea maderae ), and in crustaceans, there is SQRNFLRFamide/ TQRNFLRFamide. The FMRF motif is still present in an extended version in met-enkephalin YGG FMRF. The fact that from a precursor protein several identical copies of thyrotropin-releasing hormone or different peptides such as in proopiomelanocortin can be synthesized is equally transferred from the most primitive animals.
Some functions which are controlled in humans by hormones other than neuropeptides are regulated in early animals by neuropeptides. The calcium level maintained in vertebrates by vitamin D3 and its derivatives is regulated—for example, in earthworms (Eisenia fetida )—by neuropeptides. Although vitamin D3 could perhaps be used in earthworms, as described earlier, it is not used for calcium homeostasis . One could argue that earthworms do not get enough UV light to isomerize dehydrocholesterol in sufficient quantities (see Sect. 6.11).
13.3 Evolution of Glycoprotein Hormones
With the identification of the GPa2–GPb5 heterodimer in flies (Sudo et al. 2005), it has become obvious that glycoprotein hormones have an ancient origin. Thyrotropin-releasing-hormone-like proteins have been found in different invertebrates: for example, in cnidarians, mollusks, and nematodes. A leucine-rich receptor is also present throughout the metazoan kingdom. There are, however, some important differences. The fly and the human GPb5 lack the sixth pair of cysteines which stabilizes the seat belt of the β chain around the α chain (see Fig. 4.22). For this reason the association of GPa2 and GPb5 might not be as strong as that in thyroid-stimulating hormone, follicle-stimulating hormone, or luteinizing hormone. The separation of GPa1 and GPa2 from GPb5 from the β chains of thyroid-stimulating hormone, luteinizing hormone, follicle-stimulating hormone, choriogonadotropin, and their receptors occurred at the origin of vertebrates. GenBank, however, apart from arthropods and vertebrates, contains only a β-chain homolog from nematodes, and no α-chain homolog, although several species linking flies and vertebrates to the ancestor have been fully sequenced. It is worth mentioning that arthropods have another cysteine-knot hormone, bursicon (Sect. 5.5.5). Sudo et al. (2005) have demonstrated that the GPa2–GPb5 heterodimer and the bursicon heterodimer act on specific G-protein-coupled receptors (DLGR1 and DLGR2, respectively) without any cross-reaction.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree