Clinical phenotype
Implications
Pattern of spread
Non-metastatic biochemical recurrence with options for observation, salvage radiation therapy, or hormone therapy
Prognostic for survival
In order of worsening survival: lymph node-only metastases > bone metastases > visceral metastases (lung > liver)
Gleason grading
Prognostic for overall survival
Possibly predictive for sensitivity to docetaxel treatment
Neuroendocrine histology
Prognostic for poor overall survival
Lacks sensitivity to hormonal therapy
Correlated to Aurora Kinase A and n-myc amplification
May be predictive for Aurora Kinase A inhibition
Pain
Prognostic for survival
May be predictive of lack of benefit to sipuleucel-T
Anemia
Prognostic for survival
Performance status
Prognostic for survival
PSA levels
Prognostic for survival
Changes can be indicative of improvements in survival post-treatment
PSA kinetics are prognostic
High levels may be predictive of benefit to hormonal therapies and treatment response (AR pathway)
Low PSA despite mCRPC may indicate neuroendocrine prostate cancer and lack of benefit to hormonal agents
Lower PSA levels may be predictive of benefit with immunotherapy (i.e., sipuleucel-T)
Alkaline phosphatase
Prognostic for survival prior to and during therapy
May be predictive of response to 223Ra treatment
LDH
Prognostic for survival
Elevated in neuroendocrine prostate cancer
Circulating tumor cell enumeration
Prognostic for survival
Post-treatment CTC declines are prognostic with a range of therapies
Under evaluation as a surrogate biomarker
CTC biomarkers may provide predictive information linked to specific therapies
Prior Therapy Exposure
The current clinical states of CRPC remain defined by patterns of spread, but are now heavily dependent on prior therapy exposure (Fig. 2.1), an issue that was not emphasized in PCWG2 given the lack of approved therapies in 2008. For purposes of this book chapter, we will not discuss localized disease, rising PSA after local therapy (biochemical recurrence), or metastatic disease in hormone-naïve PCa. Instead, we will focus on sites of metastatic spread in CRPC and prior therapy exposure. In addition, within each CRPC clinical state, we will discuss the importance of independent prognostic factors including histologic subtype, serum and blood-based prognostic biomarkers, symptoms, and molecular alterations linked to CRPC progression.
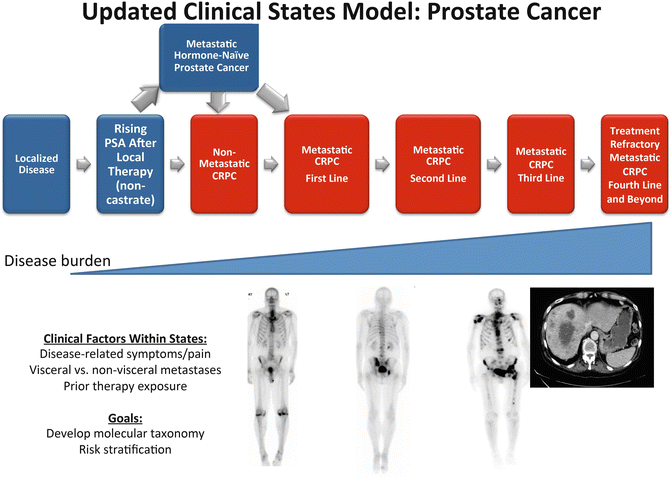

Fig. 2.1
Depiction of evolution of clinical states in prostate cancer
As more systemic treatments become available for men with CRPC, it is important to keep in mind prior therapy exposure for each patient. Prior therapy will dictate prognosis and response to the next treatment strategy, given that cross-resistance to at least several newly approved agents is expected and has been observed in the clinic. The promotion of resistance to subsequent therapy with exposure to novel hormonal agents may be a result of clonal selection, tumor evolution through mutation, or epigenetically regulated cellular plasticity and adaptation. Thus, understanding the prior exposure of a patient to a range of systemic therapies will facilitate the rational sequencing of therapies in the clinic and anticipated clinical benefit from a further line of treatment.
Both of the novel therapeutic agents abiraterone acetate and enzalutamide were tested initially in the post-chemotherapy state, after patients had progressed on docetaxel. However, it is now clear that these agents are likely more active in the pre-docetaxel mCRPC setting [14, 15], based on greater magnitudes and durability of PSA decline and disease control. For example, the PSA response rate and progression free survival for abiraterone acetate in the post-docetaxel CRPC setting is about 50 % and 8 months, while in the pre-docetaxel it is 70 % and 16 months, respectively. In the phase I–II trial of enzalutamide, 65 patients were chemotherapy-naïve and had a better PSA progression free survival [PFS] (greater than 25 % increase in PSA from baseline) compared to the 75 patients who were post-chemotherapy (median PFS not reached for pre-chemotherapy group vs. 27 weeks for post-chemotherapy group) [15]. The reasons for this may relate to lesser disease burden in the pre-docetaxel mCRPC disease state, but may also relate to cross-resistance of hormonal therapy and docetaxel. Recent studies suggest that docetaxel may have an underlying hormonal mechanism, through disruption of AR nuclear transport on microtubule shuttles. Thus, a biologic rationale has emerged that may explain clinical cross-resistance with docetaxel and hormonal therapies. Thus, appreciating prior therapy exposure is essential in predicting clinical benefit to subsequent therapy.
The phase I trial of abiraterone acetate included 19 patients who had prior ketoconazole exposure, and PSA declines of more than 50 % were noted in 9 of these 19 patients (47 %) [16]. Therefore, it appeared as though abiraterone had disease activity in CRPC even in patients who had received ketoconazole previously. Many of these men were not truly ketoconazole-resistant, and thus, it is not clear how active abiraterone would be in truly ketoconazole-resistant men. Studies of enzalutamide have been conducted in men without prior exposure to ketoconazole or abiraterone, and thus it is currently unclear whether cross-resistance exists. However, current retrospective series indicate clear evidence for a lower response rate and duration of response when enzalutamide or abiraterone are used after each other [17–19]. Therefore, the timing and sequencing of treatments can certainly determine disease response to treatment and is essential in determining clinical disease state.
Retrospective studies suggest clinical cross-resistance between enzalutamide and abiraterone acetate. One retrospective study examined 35 patients with CRPC in the post-docetaxel state, who had received abiraterone followed by enzalutamide [19]. In this study, 40 % of patients had a rising PSA as their best response to treatment with primary resistance to enzalutamide. In patients who had some response to enzalutamide (defined by at least one declining PSA value), median time to progression was only 4 months before secondary resistance to enzalutamide developed. Two other retrospective studies examined abiraterone acetate treatment after docetaxel and enzalutamide. One studied 30 patients with CRPC treated with abiraterone acetate after enzalutamide [17] and found that patients had a median time to progression of 15.4 weeks. Only three of the patients had a PSA response with abiraterone. The other retrospective study looked at 38 patients with CRPC who were treated with abiraterone after progression with both docetaxel and enzalutamide [18]. Only ten of these patients (26 %) had any PSA response, and median PFS was only 2.7 months. These data suggest that prior therapy with either abiraterone acetate or enzalutamide may promote cross-resistance with the other agent. While the mechanisms for this cross-resistance are speculative, they may involve the development of AR mutations or ligand binding domain deletion variants that become ligand independent and do not respond to either treatment.
Given the novelty of the systemic therapeutic agents, insufficient prospective data have been generated regarding cross-resistance and underlying mechanisms; biomarkers prospectively defining these mechanisms in the clinic are currently lacking. Until biomarkers of AR and androgen activity are available from clinical specimens, our current disease states remain defined by prior therapy exposure and responses.
Patterns of Spread of Metastatic CRPC
M0 Disease
The site of CRPC metastasis and the presence or absence of metastases is independently prognostic of survival. The majority of men with mCRPC progression through the M0 CRPC disease state, while only a minority of men in current US practice present with metastatic disease (3–5 %) and thus progress to mCRPC without an M0 state [1]. Non-metastatic CRPC is relatively common, with a defined, more indolent, natural history than those with metastatic CRPC [20, 21]. The M0 disease phenotype space is typically asymptomatic and prognosis is dictated by the degree of PSA elevation and the rapidity of PSA velocity or PSA doubling time (PSADT). These two factors can reliably predict the onset and timing of metastatic disease development. For example, time to bone metastases can vary by 6–12 months in men with a rapid PSADT (<4 months) vs. slower PSADT of 6–10 months [22]. Many men with M0 CRPC have an even slower rate of disease progression, with a large minority of men having no progression to metastases within 2–3 years [22]. The goal of treatment in patients with M0 disease is to prevent or delay symptomatic metastases while also increasing OS without undue toxicity. Non-metastatic CRPC or locally advanced CRPC is therefore a clear clinical state of CRPC with distinct outcomes [13]. Currently, several trials of active systemic hormonal agents (enzalutamide, ARN-509) are in phase III trials evaluating the role and clinical benefit of delaying metastasis in this setting, as opposed to the current indication of waiting for mCRPC to develop before utilizing these agents.
Recently, a trial of denosumab, a monoclonal antibody to RANK ligand (RANKL), studied time to skeletal metastasis in patients with CRPC without metastasis but with either high PSA > 8 μg/L or PSA doubling time <10 months [23]. Denosumab treatment delayed time to first skeletal metastasis from 29.5 months to 33.2 months (HR 0.84, p = 0.032) in the placebo group and significantly increased bone-metastasis-free survival from 25.2 to 29.5 months (HR 0.85, p = 0.028). However, OS was not significantly different between the two groups, the differences in time to bone metastases were not felt to be clinically meaningful, and thus denosumab has not approved for use prior to radiographically apparent metastasis. This trial, however, provides a strong dataset to study the natural history of M0 CRPC and frame future clinical trials of more active agents.
As more novel therapeutics are approved in the metastatic and post-chemotherapy setting, they are being evaluated as first-line therapy prior to docetaxel given their favorable toxicity and efficacy. For example, enzalutamide is currently being tested in metastatic CRPC patients in the pre-docetaxel setting, as well as in non-metastatic patients in the multicenter, placebo-controlled, double blind Phase 3 PROSPER trial. Thus, the M0 CRPC disease state is emerging as an important state, given the desire of patients and providers to delay as long as possible the onset of symptomatic metastatic disease.
Lymph Node Metastases
PCa metastasizes to lymph nodes in up to 10 % of patients at the time of diagnosis [24]. Men with node positive disease at the time of surgery have poorer prognosis compared to men without nodal metastases, but still have a median survival in excess of 11 years in the absence of any immediate therapy, and over 13 years with immediate hormonal therapy [25]. Of 3463 PCa patients in a retrospective analysis at the Mayo Clinic, 322 had lymph node metastasis. These patients had a 10-year PFS of 64 % with 10-year cancer-specific survival rate of 83 % [24]. A separate retrospective study in 2013 examined 369 men at Memorial Sloan Kettering Cancer Center with lymph node metastases and found their 10-year cancer-specific survival rate to be 72 % [26].
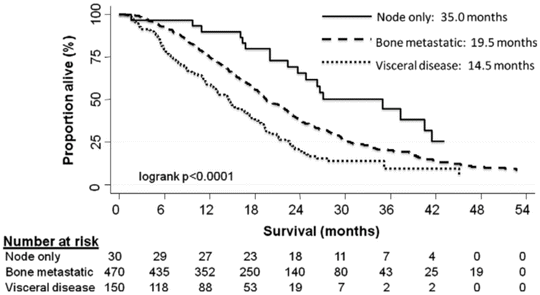
Men with lymph node-only metastasis and CRPC have a longer PFS and disease-specific survival when compared to those with bone or visceral metastasis [27]. The TAX-327 study prospectively randomized 1,006 men with mCRPC to receive either docetaxel or mitoxantrone, and OS prognosis depended on the site of metastatic spread [27] (Fig. 2.2). Patients who had lymph node-only CRPC had the best median OS of 35 months. In comparison, men with bone CRPC had median OS of 19.5 months, and patients with visceral disease had the lowest median OS of 14.5 months (p < 0.0001) [27]. Thus, lymph node-only prostate cancer and lymph node-only CRPC have relatively favorable prognosis, and clearly, the site of metastatic spread has independent prognostic significance.
Bone Metastases
Bone metastases are found in over 95 % of men with lethal CRPC [28]. The reason for poorer OS in patients with bone metastases in CRPC as compared with node-only mCRPC may lie in the different mechanisms of hematogenous vs. lymphatic dissemination of tumor cells, the formation of sclerotic bone metastases that are highly resistant to therapies, or the greater disease burden in these men. This mechanism may depend on PCa cells undergoing a phenotypic change to a more mesenchymal or osteomimetic state, developing the ability to invade blood vessels, and colonizing the bone marrow by extravasating out of the blood vessel. This process, termed epithelial-to-mesenchymal transition (EMT), may lead to the acquisition in prostate cancer of stemness properties that permit homing of the cancer to bone, and has been observed in disseminated tumor cells and circulating tumor cells (CTCs) of men with mCRPC and in preclinical models of PCa [29–33]. Chemokines, inflammation, bone stroma, circulating mesenchymal and stem-like progenitor cells, and other host factors may regulate this process, and intrinsic oncogenic programs to the PCa cell may promote bone metastases [29, 34–36]. Once the PCa cells are situated within the bone marrow niche, they interact with signaling molecules such as transforming growth factor beta (TGFβ), vascular endothelial growth factor (VEGF), and RANK ligand (RANKL) to acquire a bone-like primitive phenotype [29], compete for the niche of hematopoietic stem cells and lead to progressive bone marrow failure [29, 37]. This plasticity or dual epithelial–mesenchymal nature of CRPC has been observed in bone metastases and CTCs from men with CRPC, indicating the importance of molecular pathways regulating stemness, differentiation, and plasticity during the development of bone metastasis [30, 33, 38]. The clinical significance of this bone-homing program is obvious once bone metastases have developed; however, there is a lack of validated biomarkers in localized prostate cancer that can predict for the onset of bone metastases.
Patients with CRPC who develop bone metastases can derive benefit from therapy that targets the bone microenvironment, including bisphosphonates such as zoledronic acid [39], RANKL inhibitors such as denosumab [40], and the radiopharmaceutical radium-223 [41]. The denosumab trial randomized 1,904 patients with CRPC metastatic to bone to treatment arms of either denosumab or zoledronic acid. The time to first skeletal event was the primary endpoint. Patients treated with denosumab had median time to the first skeletal event of 20.7 months, an improvement of 3.6 months when compared to 17.1 months for those treated with zoledronic acid (hazard ratio 0.82, p = 0.0002) [40].
A novel radioisotope, radium-223 chloride, was recently approved by the FDA to treat PCa bone metastases. The ALSYMPCA phase III trial enrolled 921 patients who had only bone metastases and were either post-docetaxel or pre-docetaxel due to medical fitness or patient refusal [42]. These patients were given radium-223 (50 kBq/kg intravenously every 4 weeks) for 6 treatments or placebo. Patients on the radium-223 arm had a higher median OS of 14.9 months compared to 11.3 months in patients treated with placebo (p < 0.001) and demonstrated longer time to first skeletal event at 15.6 months compared to 9.8 months, respectively (p < 0.001) [42]. Thus, bone metastases are a clinical disease state that determines treatment decision-making and the use of specific bone-targeting agents that are not known to exhibit activity outside of the bone. Future work to define biomarkers that can reliably predict for the development of bone metastases are needed in order to develop trials of systemic agents for the prevention of bone metastases.
Visceral Organ Metastases
When CRPC metastasizes to visceral organs such as lungs and liver, the disease course is often more aggressive than CRPC with metastasis to lymph nodes and bones (Fig. 2.2). Men with lung or liver metastases appear to benefit from both novel agents such as enzalutamide and abiraterone acetate, as well as traditional chemotherapy agents such as docetaxel. However, patients have much lower rates of response and survival once visceral metastases, particularly liver, occur. Patients with visceral metastases are currently restricted from receiving certain systemic therapies, such as radium-223, per the FDA label. In addition, current NCCN guidelines do not recommend the use of sipuleucel-T in men with liver metastases, given that these men have a poorer prognosis, and men with visceral metastases were excluded in the phase III trial of sipuleucel-T.
Data from the TAX-327 trial demonstrated that men with metastatic CRPC to the liver had the poorest prognosis (10 months), as compared to men with lung metastases (14.4 months) [43]. This observation is reflected in published nomograms in this population, with strong negative impact on survival in the presence of liver metastases [44, 45]. A recent poster presentation at the 2013 ASCO annual meeting also showed that patients with metastatic CRPC to lungs alone had a median OS of 15.5 months, compared to 7.7 months in those patients who had metastatic CRPC to liver or liver + lung [46]. Thus, the pattern of visceral spread is also an important consideration in determining clinical phenotype in mCRPC.
Histological Categories of CRPC
The histology of PCa is commonly based on their origin from glandular-forming epithelial cells. While the cell of origin of PC is debated and may be the basal cell of the prostate, a subset of a luminal cell population, or both depending on context, typical prostate cancer is adenocarcinoma and consists of glandular architecture with varying degrees of loss of differentiation. Cells in the normal male prostate do not normally proliferate, but are able to survive repeat bouts of castration and regeneration. However, over time, with cumulative mutations and epigenetic lesions, PCa cells acquire the ability to continuously proliferate despite AR ablation, and acquire an invasive phenotype [3]. The Gleason grading system was initially described in 1966 [47], and was validated as a prognostic measure at the Minneapolis Veterans Administration in 1974 [48]. This grading system was updated in 2005 to correspond more closely with patient outcome [49]. As a score of nuclear polymorphism, glandular disruption, disease heterogeneity, basement membrane disruption, and de-differentiation of prostate cancer, the Gleason score reflects the aggressiveness of the individual PCa. The Gleason sum continues to be independently prognostic for survival in the CRPC setting, as demonstrated repeatedly in multiple nomograms for prostate cancer survival [44, 50]. The Gleason sum has also been found recently to be potentially predictive for treatment response and sensitivity to treatment with docetaxel in the TAX-327 trial [51]. In this analysis, higher tumor grades (Gleason score ≥7) had a more pronounced survival benefit from treatment with docetaxel than with mitoxantrone [51]. Thus, higher Gleason grading can be both prognostic for survival and potentially predictive for a greater magnitude of treatment benefit from docetaxel. While this requires confirmation, tumor grade may provide a surrogate biomarker for genomic instability, proliferation, and de-differentiation that may be exploitable in the clinic and provides independent prognostic information.
Adenocarcinoma is the main histologic form of PCa, accounting for 95 % of all PCa [52]. The remaining 5 % is made up of ductal, mucinous, small cell, and anaplastic carcinoma, each of which comprises a more aggressive variant that may progress to CRPC more rapidly and behave as high grade tumors. In the national SEER database, there is an incidence of 61 cases per 10,000 people per year for mucinous carcinoma, 49 cases per 10,000 people per year for ductal carcinoma, and 35 cases per 10,000 people for anaplastic or neuroendocrine carcinoma [53]. Mucinous carcinoma has similar 5-year OS to adenocarcinoma (75.1 vs. 76.5 % respectively), and ductal carcinoma has a slightly more aggressive disease course, with a 5-year OS rate of 61.7 % [53].
Contrastingly, neuroendocrine prostate cancer (NEPC) is much more aggressive and has a 5-year OS rate of only 12.6 % [53]. NEPC can arise either de novo with a low serum PSA level, obstructive symptoms, and often distant metastases at the time of diagnosis, or more commonly, NEPC emerges as a secondarily resistant subtype after prostate adenocarcinoma treatment. NEPC is independent of AR signaling and can be a mechanism of castration resistance (Table 2.2). NEPC often overexpresses chromogranin A and synaptophysin, biomarkers which are detectable in serum [54]. Many molecular alterations accumulate in NEPC, including amplification of Aurora Kinase A and N-myc [55], overexpression of EZH2 [55], loss of Rb [56], and activation of the PI3 kinase pathway [57]; these represent potential therapeutic targets. During therapy with an Aurora Kinase A inhibitor, these tumors revert to adenocarcinoma features, indicating histologic plasticity [55]. This phenomenon mimics what is observed in the reversible transitions between small cell and non-small cell lung carcinomas [58]. This toggle effect raises the possibility that prostate cancer cells have an inherent plasticity to change histological subtypes when evading treatment pressures and imply that the current or prior histologic appearance of a CRPC patient’s tumor may be important clinically.
Table 2.2
Prostate cancer categories based on androgen and AR dependency
Categories of prostate cancer based on androgen ligand and androgen receptor activity | Examples |
|---|---|
1. Androgenic ligand dependent, androgen receptor dependent | Wild-type AR, AR mutants, AR amplification, autonomous androgen synthesis |
2. Androgenic ligand dependent, androgen receptor co-opted | Glucocorticoid receptor, other promiscuous ligands |
3. Androgen independent, androgen receptor dependent | AR variants, AR deletions, non-canonical AR activation |
4. Androgenic ligand independent, androgen receptor independent | Neuroendocrine prostate cancer, basal/stem-like prostate cancer, EMT? |
NEPC correlates with poor clinical prognosis [53]. A series of 21 patients with NEPC were treated with cisplatin-based chemotherapy active in small cell carcinoma of the lung, with a resulting median OS of 9.4 months (range 1–25 months) [59]. A phase II trial of cytotoxic chemotherapy in 120 neuroendocrine prostate cancer patients subsequently treated patients first with carboplatin and docetaxel (CD), followed by cisplatin and etoposide (EP) [60]. Primary endpoints included response rates and time to progression. Only 74 patients were able to undergo treatment with both regimens. Of these men, 50 % had benefit from both regimens, 34 % responded to CD but not to EP, 9 % responded to EP but not to CD, and 7 % did not respond to either regimen. Median OS was 16 months [60]. Therefore, NEPC carries a poor prognosis despite multi-agent platinum chemotherapy. The emerging biologic differences inherent in NEPC transformation however suggest that molecularly targeted therapies, likely in combination, may have specific activity in this disease. The Aurora Kinase inhibitor MLN-8237 is currently in a phase II trial which is actively enrolling patients with NEPC to evaluate drug efficacy and predictive biomarkers (ClinicalTrials.gov number NCT01799278). Thus, NEPC represents a distinct CRPC histologic and molecularly defined entity. Current barriers, however, include defining the clinical characteristics of men with CRPC who have this NEPC genotype.
Symptom as Indicative of Clinical State of CRPC
Pain
CRPC patients often have pain but can have metastatic disease without the presence of pain. Pain often arises when CRPC metastasizes to bone and is often described as dull and achy, migratory, and sometimes progressing to sharp pain in axial more than appendicular regions of the skeleton. However, many patients develop multiple sites of metastatic disease in the absence of pain. In the TAX-327 trial, half of the patients who had multiple areas of high uptake on bone scan did not have pain, while the other half required opioid medications to control pain (unpublished data). Systemic therapy may also induce clinical response in pain without PSA response [61, 62]. The measurement of pain should be objectively ascertained, using reliable and validated surveys or patient reported outcomes. Cancer-specific pain requiring opioid analgesia is considered significant pain in current prognostic models of CRPC.
Although pain does not correlate with presence of metastases, clinically significant pain is an independent prognostic indicator for OS and is included in current nomograms for survival in CRPC [44, 45]. In TAX-327, patients who experienced pain relief with treatment had a higher median OS of 18.6 months, compared to 12.5 months for patients who did not experience pain relief [61]. Similarly, a large analysis of NCI cooperative group trials of men with CRPC also demonstrated that more severe pain was associated with shortened survival (17.6 vs. 10.2 months for men with low vs. high pain scores [63]). These results have been repeated in studies of abiraterone acetate as well, and therefore novel hormonal agents can also change this clinical manifestation of disease with subsequent improvement in prognosis [64]. Pain and symptomatic CRPC is currently included in the approved USFDA labels and NCCN guidelines for sipuleucel-T (absence of significant symptoms) and radium-223 (presence of symptoms). Thus, the pain phenotype connotes a meaningful impact on outcomes, quality of life, and treatment selection of a man with CRPC.
Pain relief and prevention is clinically significant. A response in pain led to the FDA approval of mitoxantrone chemotherapy in CRPC [65], and the prevention of skeletal related events led to FDA approvals for zoledronic acid and denosumab [39, 40]. Ongoing phase III trials of the dual VEGFR2/c-MET inhibitor, cabozantinib, in patients with CRPC also have durable pain palliation as an endpoint under evaluation for regulatory approval, provided these improvements are associated with improved survival and are substantial and clinically meaningful. Thus, pain is clinically significant and is prognostic for inferior survival outcomes. Treatment selection may also depend on pain severity and response, as several of the systemic therapies in the treatment sequence may be selected due to their intended effect on clinical pain.
Anemia
Anemia-related fatigue is a complicating symptom in a subset of patients with CRPC [66, 67]. Anemia can be attributed to multiple causes, including anemia of chronic disease, androgen-deprivation therapy, chemotherapy toxicity, renal disease, disseminated intravascular coagulation (DIC), blood loss, or bone marrow infiltration of prostate cancer cells. Bone marrow infiltration results from prostate cancer cells acquiring a stem-like phenotype and taking over the bone marrow niche of hematopoietic stem cells [29]. Anemia can therefore indicate bone marrow infiltration and ultimately bone marrow failure, which contributes directly to patient mortality.
Anemia in men with CRPC is an independent prognostic marker for worse prognosis; it has been shown in multivariate analysis to be an important symptom in all published nomograms for survival in metastatic CRPC [44, 45, 50, 66, 68]. From the TAX-327 database, anemia contributed to a median OS of 14.9 months compared to 21.7 months in patients without anemia (Fig. 2.3).
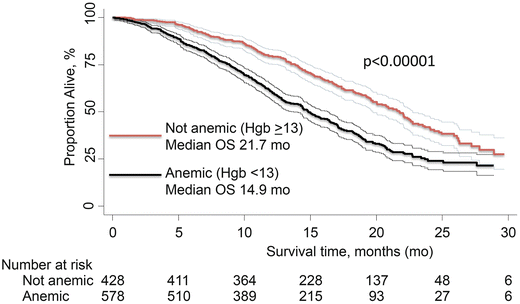

Fig. 2.3
Kaplan–Meier overall survival estimates for patients with mCRPC in the TAX-327 trial with anemia (hemoglobin <13 g/dL) vs. patients without anemia (hemoglobin ≥13 g/dL). Data is based on Armstrong et al. [44]
Although erythropoietin to stimulate red blood cell production has been shown to increase hemoglobin levels and increase quality of life in some patients with CRPC [69, 70], a Cochrane meta-analysis ultimately showed that erythropoietin increases rates of hypertension, thrombocytopenia, and venous thromboembolism in patients with solid tumors, and may accelerate tumor progression in several cancer types [71]. Therefore, erythropoietin is only given if the severe anemia is caused by chemotherapy, and then only after an informed consent discussion, using a hemoglobin level of less than 10 g/dL as the indication for treatment initiation and continuation of treatment only if the hemoglobin remains less than 10 g/dL. In the circumstance of marrow infiltration by tumor, anemia generally improves with effective PCa therapy, including chemotherapy and hormonal therapies, which may also reverse coagulopathy.
Performance Status
The Karnofsky Performance Status (KPS) has been well established as an important prognostic factor in multiple nomograms and multivariate analyses of survival in men with mCRPC [44, 45, 50, 68]. KPS often depends on patient state at time of presentation. KPS can be affected by comorbidity and prior medical conditions, but also can reflect other symptoms such as pain, anemia, emotional distress and mood changes after a cancer diagnosis. Thus, functional status is of utmost importance both in determining prognosis and in leading the patient and provider to selecting specific systemic therapies. However, KPS remains a crude measure of functional activity in men with asymptomatic mCRPC or M0 CRPC or earlier disease states. Other tests of functional activity, such as exercise tolerance or geriatric functional assessment scales, may provide a greater assessment of physiologic reserve. The ability of novel hormonal agents such as enzalutamide and abiraterone acetate, as well as the radiopharmaceutical radium-223, to improve survival in men with metastatic CRPC and impaired performance status opens up several important treatment options for most men and marks an important milestone in the history of PCa treatment [14, 42, 72]. However, these hormonal agents may also impair functional status in men with asymptomatic CRPC due to loss of lean muscle mass and gain of metabolic derangements and fat mass [73]. Thus, attention to functional status in CRPC is critical in the assessment of treatment selection, response, and toxicity.
Biomarkers Indicative of Clinical States of CRPC
Multiple serum or blood-based biomarkers have prognostic significance in the progression of clinical states of CRPC, including PSA, alkaline phosphatase (AP), lactate dehydrogenase (LDH), albumin, and CTCs [74]. With the exception of CTCs, most of these biomarkers have been shown in nomograms with multivariate analyses to be independently prognostic of patient survival from CRPC as well as have implications for treatment selection, and CTCs have also been independently associated with mortality in men with metastatic CRPC [75]. For example, AR signaling regulates PSA production, and increasing PSA levels may indicate AR pathway dependence, while low PSA levels despite metastatic progression such as in NEPC indicates androgen receptor independent prostate cancer (ARIPC). Patients with intact wild-type AR are dependent on AR signaling and appropriate for AR-targeting therapies; however, those patients who develop splice variants of AR with C-terminal deletions would not benefit from AR-targeting agents. In addition, patients can further develop ARIPC, where cellular proliferation is no longer dependent on AR signaling. In these cases of ARIPC, cytotoxic agents are potentially more beneficial. Bone biomarkers such as AP may reflect the tumor burden within bone and potential benefit with bone-targeted therapies.
Prostate Specific Antigen
PSA was developed to screen and diagnose PCa, as well as to track the response of PCa to therapies. Although PSA is controversial as a screening tool for PCa, PSA is widely considered to be a useful tool to monitor disease recurrence and response to local and systemic therapies. PSA levels and PSA kinetics are independently prognostic for survival across all PCa disease states, including M0 and M1 CRPC [20, 45]. Declines of least 30 or 50 % in PSA during treatment with chemotherapy are highly associated with improvements in OS [61]. For example, in the TAX-327 trial, men who attained PSA reduction at least 30 % during the first 3 months of chemotherapy had a median OS of 21.6 months, compared with 13.0 months for patients who had less than 30 % PSA decline, and this threshold of decline had the greatest association with survival, despite relatively modest surrogacy [61]. A phenomenon that reduces the ability to associate PSA declines with survival outcomes is the transient rises that occur during the initiation of systemic therapy. Transient PSA rises can occur in 15–20 % of men with CRPC in the first 3–4 months of chemotherapy, can be of substantial magnitude (60–400 % rises have been reported), and have usually resolved by cycles 3–4 of chemotherapy [62]. These transient rises in PSA do not confer prognostic significance, and clinicians should continue treatment through isolated changes in PSA during the first few cycles of systemic treatment.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree