© Springer Science+Business Media New York 2014
Fred Saad and Mario A. Eisenberger (eds.)Management of Castration Resistant Prostate CancerCurrent Clinical Urology10.1007/978-1-4939-1176-9_2323. Evidence-Based Therapeutic Approaches for mCRPC Patients: Rational Sequence of Standard Treatment Options and Design for Future Drug Development
(1)
Icahn School of Medicine at Mount Sinai, Tisch Cancer Institute, New York, NY, USA
(2)
Division of Hematology and Medical Oncology, Icahn School of Medicine at Mount Sinai, Tisch Cancer Institute, One Gustave L. Levy Place, 1128, New York, NY 10029, USA
Keywords
prostate cancercastration resistancemetastaticoptimal sequencinghormone refractoryIntroduction
Over the last 3 years, at least five new anticancer therapies have been approved for the treatment of metastatic castration-resistant prostate cancer (mCRPC): cabazitaxel, sipuleucel-T, abiraterone, enzalutamide, and radium-223. These treatments—along with docetaxel, which was approved in 2004—represent the current list of agents that have demonstrated a survival benefit in patients with mCRPC. Each is capable of extending survival for individuals with metastatic prostate cancer that is resistant to primary androgen deprivation therapy; however, none of these treatments has been shown to be curative. Therefore, the state of the art treatment for mCRPC remains associated with progression after multiple different therapeutic regimens. Appropriately sequencing and possibly combining these treatment regimens will be crucial for maximizing survival and minimizing adverse events.
Unfortunately, there is currently little prospective evidence for when to use each available therapy. Given the rapid pace of drug discovery, each of the recent approvals came as a result of comparison either to placebo, or—in the case of cytotoxic chemotherapy—randomization against mitoxantrone, a chemotherapy drug that does not improve survival. Clinical trials of new drugs in sequence logically come after trials that establish their initial safety and efficacy. Therefore, it is expected that studies on drug sequencing and combinations will lag behind their single-drug counterparts. Moreover, studies of multiple drugs in sequence are clearly more difficult to complete than trials of single agents tested against placebo or existing treatments. Finally, preclinical models of specific sequential therapies are rarely performed and often do not demonstrate predictive value. For instance, it is extremely difficult to model a course of chemotherapy followed by immunotherapy in current animal models.
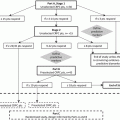
Existing treatments for mCRPC can be classified into one of four broad groups based on their mechanisms of action: (1) androgen signaling pathway inhibitors (2) immunotherapy (3) cytotoxic chemotherapy, and (4) bone-directed therapies. Though each has been shown to improve survival, all of these approaches eventually become ineffective as the tumor cell population develops resistance. Since treatment itself may foster tumor resistance, this can consequently impact the value of sequential therapies. It is critical to understand these changes in tumor sensitivity in order to know which therapies (if any) should precede others.
Additionally, we do not yet understand which drugs should be given together to create additive or synergistic combinations. Effective combinations may be found within classes, such as two anti-androgenic agents, or between classes, such as a bone-targeted therapy concurrent with an androgen pathway inhibitor. In either case, combinations seek to block or prevent paths of drug resistance and to create more effective ways to induce tumor cell death.
In studying these new therapies, it has also become clear that prostate cancer can be very heterogeneous in its initial presentation, manner of progression, and response to therapy. For instance, there are clinical trials in which the treated group did not benefit enough to improve overall survival, but in which some individuals have been shown to have dramatic responses. It is imperative to understand what distinguishes good and poor responders for a particular therapy in order to limit unnecessary toxicity and also to achieve the best response to a given therapy, even if only in a subset of patients with mCRPC. Biomarkers are emerging that may help predict survival [1]. The next step will be to uncover biomarkers that predict the most effective and safe treatment response and thus sequence of therapy.
Recent advances in drug development have improved the outlook for patients with mCRPC, though the disease remains fatal. Over the next few years, trials of drugs in sequence and in combination will be conducted to contextualize new therapies within the array of treatment options. Additionally, we must develop better means of understanding individual tumors and selecting the best responders to specific treatments. In this chapter, we will review the available data to help tailor drug selection and timing for men with mCRPC.
Optimal Timing of Immunotherapy
Sipuleucel-T is the first immune therapy approved for treatment of mCRPC or indeed any cancer. In the IMPACT study, sipuleucel-T demonstrated a 4.1 month improvement in overall survival in men with asymptomatic or minimally symptomatic mCRPC [2, 3]. This population was selected because the immune response is thought to act over months or years to inhibit tumor progression. Early use of sipuleucel-T in mCRPC could thus hopefully provide the most benefit. In the IMPACT study, sipuleucel-T paradoxically did not show a significant improvement in time to progression or clinical response compared with placebo. In fact, sipuleucel-T rarely induces significant PSA declines and does not appear to palliate symptoms of metastatic disease. Therefore, patients with symptomatic disease usually require treatments such as chemotherapy or androgen pathway drugs that can more effectively improve cancer-related symptoms.
The optimal timing for the initiation of sipuleucel-T in patients with mCRPC remains uncertain. Schellhammer et al. recently reported a post-hoc analysis of the IMPACT trial in which patients were grouped into quartiles based on their baseline PSA level on study entry [4]. This analysis demonstrated that both the absolute survival difference and the hazard ratios for survival were significantly improved in the lower quartiles of PSA. Since baseline PSA represents a measure of disease burden, this study suggests that the maximal benefit of sipuleucel-T may be when the PSA and thus overall disease burden with mCRPC is lowest.
Giving sipuleucel-T earlier in asymptomatic mCRPC may also have other advantages. Sipuleucel-T has a favorable side-effect profile, with just 6.8 % of patients in the IMPACT trial reporting adverse events of grade 3 or more, compared to 1.8 % in the placebo group [3]. Side effects were most commonly flu-like symptoms, such as fever, chills, and rigors. Sipuleucel-T’s toxicity compares favorably to the other treatments for mCRPC since it may preserve a patient’s quality of life compared with treatments like chemotherapy, which have been associated with more adverse effects.
Another important factor to consider when sequencing sipuleucel-T and other immune therapies is whether the patient is concurrently being treated with drugs that could suppress the immune system. Since sipuleucel-T works by targeting the immune system against the tumor-specific antigen, prostatic acid phosphatase, it requires an intact immune system to be effective. Abiraterone acetate is given with prednisone, which could have a suppressive effect on immunotherapy. Chemotherapy is also often accompanied by corticosteroids, both of which could be similarly problematic for ongoing immune therapy.
The interaction between chemotherapy and sipuleucel-T is poorly understood. No randomized trials have studied the two therapies concurrently or in a specific sequence. On one hand, it is understood that chemotherapy induces apoptotic cell death—a form of destruction that is not regarded as immunogenic. Additionally, chemotherapy is known to be immunosuppressive. However, a recent study suggests that tumor cells being destroyed by chemotherapy may have important interactions with the immune system that could enhance response to immunotherapy [5].
Further studies are required to definitively place sipuleucel-T in sequence with other available therapies for mCRPC. However, our current knowledge allows us to safely give sipuleucel-T to patients with metastases who have progressed on first-line hormone therapies yet do not have symptoms that warrant treatment with drugs which induce immediate anticancer responses, such as chemotherapy or androgen pathway inhibitors. In this population, patients receiving sipuleucel-T have an overall survival benefit compared to patients receiving placebo, with few side effects—an ideal cost-benefit balance for the asymptomatic or minimally symptomatic mCRPC patient.
When Should Androgen Pathway Therapies Be Used Relative to Chemotherapy?
While sipuleucel-T has not been shown to palliate symptomatic mCRPC, androgen pathway drugs, cytotoxic chemotherapy, and radium-223 have all been associated with both a survival benefit and the ability to induce symptomatic responses in patients with mCRPC. Given the recent approval of radium-223 in May 2013, little is yet known about exactly when it should be used in practice. However, we now have several years of experience and more data on the relationship between androgen pathway drugs and chemotherapy. A crucial dilemma lies in the sequencing of these two types of treatments. In 2004, docetaxel was the first agent to show an improvement in survival for patients with mCRPC in phase III clinical trials [6, 7]. Over the past 3 years, abiraterone was approved for use after chemotherapy and then in chemo-naïve patients [8, 9]. Similarly, enzalutamide was approved for use after chemotherapy [10], with an ongoing phase III trial in chemo-naïve patients recently reported to have met its primary endpoint (NCT01212991). While we do not have a definitive sequence of chemotherapy and androgen pathway agents unless a randomized trial directly studies this question, existing data provide some clues as to a rational sequence of these two key classes of drugs.
Two registrational phase III trials compared abiraterone plus prednisone to prednisone alone. COU-AA-301 enrolled patients who had previously received docetaxel chemotherapy [8] while COU-AA-302 enrolled patients naïve to chemotherapy [9]. While it is not possible to make definitive conclusions by comparing phase III trials, there is a suggestion from these studies that there may be an advantage to giving abiraterone before chemotherapy. While hazard ratios for survival were similar pre- and post-chemotherapy, both the absolute benefit and hazard ratio for radiographic progression-free survival (rPFS) were significantly better in the pre-chemotherapy trial. rPFS was improved by 8 months (HR 0.53) in patients given abiraterone before chemo and 2 months (HR 0.65) in patients give abiraterone after chemotherapy [8, 9, 11]. While not definitive, there is a suggestion that the maximal benefit from abiraterone may be seen if it is used before chemotherapy.
Additionally, abiraterone may be a better choice earlier in the disease course based on its more favorable toxicity profile. Approximately a third of chemo-naïve patients on abiraterone plus prednisone reported serious adverse events [9]. Peripheral edema, arthralgia, and fatigue were among the most common adverse events in abiraterone treated patients. Docetaxel, on the other hand, is associated with an increased incidence of more serious side effects. Twenty-six percent of patients treated with docetaxel and prednisone every 3 weeks experienced serious side effects such as neutropenia, diarrhea, dyspnea, sensory neuropathy, peripheral edema, and fatigue. Abiraterone plus prednisone increased the median time to initiation of cytotoxic chemotherapy by 50 % (p < 0.001) [9]. Thus, using abiraterone therapy prior to chemotherapy could be a means of maximizing progression-free survival in mCRPC, while delaying the use of potentially more toxic chemotherapeutic agents.
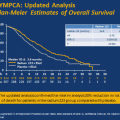
Survival analysis of the two phase III abiraterone trials demonstrates several interesting findings. After docetaxel chemotherapy, the hazard ratio for overall survival for the abiraterone arm was 0.75 after a median follow-up of 20.2 months [11]. In the study of chemo-naïve patients receiving abiraterone, the hazard ratio for overall survival was also 0.75 [9]. This identical hazard ratio for the two groups suggests that chemotherapy may not have influenced resistance rates to subsequent androgen pathway therapies. Obviously, there are important limitations to this type of comparison, including the fact that the two studies enrolled groups of patients that were substantially different. Patients treated with abiraterone after completing chemotherapy were clearly more ill and advanced in their disease state. Patients in the pre-chemotherapy study survived substantially longer regardless of treatment with abiraterone. In addition, 46 % of the chemotherapy-naïve patients went on to receive docetaxel or cabazitaxel after completing abiraterone [9], which likely conferred an additional survival benefit. Based on existing data, one cannot conclude with certainty that abiraterone would have a differential effect on overall survival if used before or after docetaxel chemotherapy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree